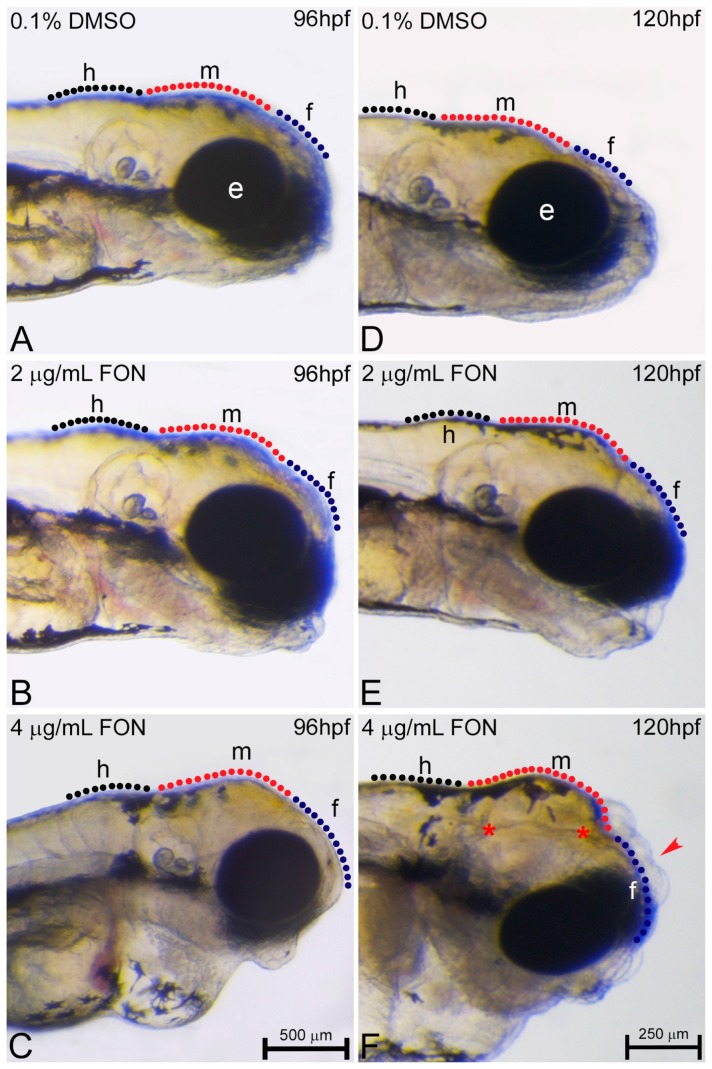
Figure 6.
Triadimefon exposed embryos display cranial defects. Representative morphological evaluation of embryos (A–C) at 96 hpf and (D–F) 120 hpf. 2 μg/mL triadimefon treated embryos present normal brain development at 96 hpf (B). At 4 μg/mL the first sings of cranial forebrain-midbrain morphological alterations are observed (C, 89/89 embryos). Morphological alterations at 2 μg/mL triadimefon treated embryos at 120 hpf include a decrease of the average brain size with the forebrain developing a compacted-like form (66/80 embryos), whereas midbrain development is slightly delayed (64/80) (E). A more pronounced phenotype is observed at the same stage with 4 μg/mL triadimefon (F). Deformities were of high severity and comprised of severely hypoplastic forebrain (red arrowhead in F, 80/89), decreased midbrain size lacking forebrain-midbrain boundary (89/89) and delay of hindbrain development (89/89). A high proportion of embryos also exhibited severe cleft of the anterior nervous system (red asterisks, 74/89). Dotted blue, red and black lines in A–F map the forebrain, midbrain and hindbrain cranial structures respectively. FON, triadimefon. Embryos in A–C are shown to the same scale (bar = 500 µm in C), while embryos in D–F are shown to scale (bar = 250 µm in F).