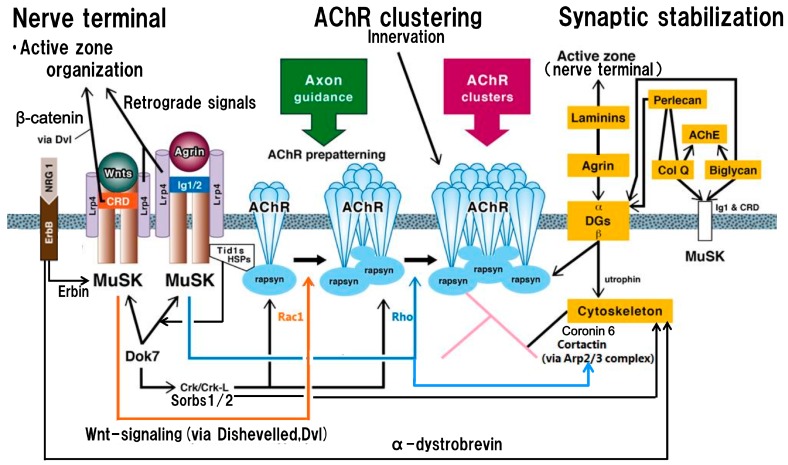
Figure 1.
Schematic presentation of the postsynaptic structure and function on the basis of acetylcholine receptor clustering centered on agrin- and Wnt-signalings, trans-synaptic communication and synaptic stabilization. In the postsynaptic membrane, nicotinic acetylcholine receptors (nAChRs) are aggregated in the non-innervated stage via Wnts-MuSK cysteine-rich domain (CRD)-dishevelled protein signaling pathway to form nAChR microclusters (AChR prepatterning: located in the central part of the muscle membrane where incoming axons are guided) (orange line with arrow; via activation by Rac1), and in the innervated stage via agrin-MuSK immunoglobulin-like domains 1 and 2 (Ig1/2) signaling pathway to form full-sized nAChR clusters (blue line with arrow; via activation by Rho). The nAChR clusters in non-innervated and innervated stages are anchored by rapsyn (immobilized by heat-shock proteins, HSPs, including tumorous imaginal disc 1 short form, Tid1s, which belongs to HSP40 family) at the postsynaptic membrane. The kinase activity and subsequent downstream signalings by the intracellular tyrosine kinase domain of MuSK, located after the transmembrane segment, are crucial for the formation and maintenance of the neuromuscular junction. The low-density lipoprotein receptor-related protein 4 (Lrp4) plays an essential role in agrin- and Wnts-signaling pathways as the receptor for both signals. In the molecular structure of Lrp4, the first propeller domain interacts with agrin; in the third β-propeller domain, its edge part mediates the MuSK signaling and its central cavity mediates the Wnt signaling. As the trans-synaptic communication, the signaling mediated by Wnts-MuSK CRD contributes to the retrograde signal from muscle to nerve (Wnt canonical pathway via dishevelled protein (Dvl) and β-catenin), leading to presynaptic differentiation to localize active zone proteins for efficient synaptic transmission (left upper part). Others participating in the trans-synaptic communication are reviewed in detail in the text; among them, the retrograde signal of Lrp4 originates from its eight low-density lipoprotein a (LDLa) repeats to induce clustering of synaptic vesicle and active zone proteins [60] (left upper part). In addition, as shown in the right part (a part of synaptic stabilization), Laminins conduct the retrograde signal to firm the active zone architecture for sufficient synaptic transmission. Intracellularly, Dok7 (downstream of kinase 7) and neuregulin 1 (NGR1)-ErbB receptor (receptor tyrosine kinase of EGF, epidermal growth factor, receptor family) (mediator: Erbin) interaction activate intracellular MuSK tyrosine kinase domain for nAChR cluster formation (left part); also, they contribute to postsynaptic stability via respective downstream effectors. The mediator for Dok7 signal is Sorbs1/2 (downstream effectors of CT10 regulators of kinase (Crk/Crk-L)); the mediator for NGR1-ErbB receptor interaction is α-dystrobrevin (both are indicated by long black lines from left to right with arrows). As shown in the right part (yellow frames) of the figure, the stability of the neuromuscular junction including AChR clusters, MuSK and acetylcholinesterase (AChE) in the postsynaptic membrane is modulated by the extracellular matrix proteins (collagen Q (Col Q), perlecan, biglycan (glycosaminoglycan-binding form) and dystroglycans (DGs)), which participate in cytoskeletal dynamics. In addition, the postsynaptic structure is stabilized by the laminin-network including laminins, muscle agrin and DGs. The transmembrane dystroglycan (β-type) binds to rapsyn for anchoring AChR clusters at the postsynaptic membrane and also link utrophin to cytoskeleton for synaptic stability. Additionally, Col Q (C-terminus) and biglycan (non-glycanated form) bind both MuSK extracellular domains (Ig1 and CRD), leading to their implication in reinforcing a functional bridge between the agrin-signaling and the Wnt-signaling. Cortactin acts as a tyrosine kinase substrate and also a regulator of actin polymerization via actin-related proteins 2/3 complex (Arp2/3 complex); its tryrosine phosphorylation is enhanced by agrin/MuSK signaling (as indicated by blue line from central to right with arrow). Coronin 6 contributes to firm nAChR clustering via the modulation of actin dynamics.