Table 1.
Study of antibodies against muscle specific tyrosine kinase (MuSK) extraceullar segment (immunoglobulin-like 1 and 2 (Ig1/2) domains and cysteine-rich domain (CRD)): clinical and immunological profiles of 33 patients positive for MuSK (extracellular full-length) antibodies and negative for nicotininc acetylcholine receptor (AChR) antibodies.
| Groups (Number of Patients) | 10 Patients | 23 Patients |
|---|---|---|
| Antibodies against recombinant segments | Anti-Ig1/2-positive Anti-CRD-positive | Anti-Ig1/2-positive Anti-CRD-negative |
| Age at onset | Age (years): 22–75 | Age (years): 6–80 |
| Gender | Female: 8/Male: 2 | Female: 15/Male: 8 |
| MuSK antibody titers determined by standard RIA (control, <0.05 nmol/L) | 6.08–131.40 | 5.32–45.75 |
| MG severity (MGFA grades) | ||
| IIa | 0 | 3 |
| IIb | 0 | 7 |
| IIIa | 0 | 2 |
| IIIb | 4 | 1 |
| IVa | 0 | 0 |
| IVb | 0 | 0 |
| V | 6 | 10 |
| Immunoblots of purified recombinant proteins of human MuSK extracellular segments | 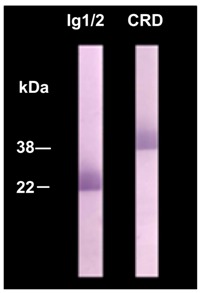 |
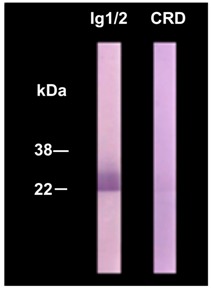 |
MGFA: Myasthenia Gravis Foundation of America. Figures indicate numbers of the patients subject to each item. The study collected 43 anti-AChR-negative patients, 10 of whom were negative for antibodies determined by both the standard radioimmunoassay (RIA, extracellular full-length of MuSK used as antigen) and the present study (Ig1/2 domains and CRD of MuSK used as antigens). Immunoblotting was done using purified recombinant protein of human MuSK Ig1/2 domains and CRD. Immunostained reactivity was tested with serum samples (1:500 dilution) from myasthenia gravis patients at 5 μg recombinant protein/lane; 22kDa and 38 kDa immunostained bands were visualized as anti-Ig1/2 domains and anti-CRD, respectively ; these were confirmed by using mouse anti-human monoclonal antibodies, respectively [65].
