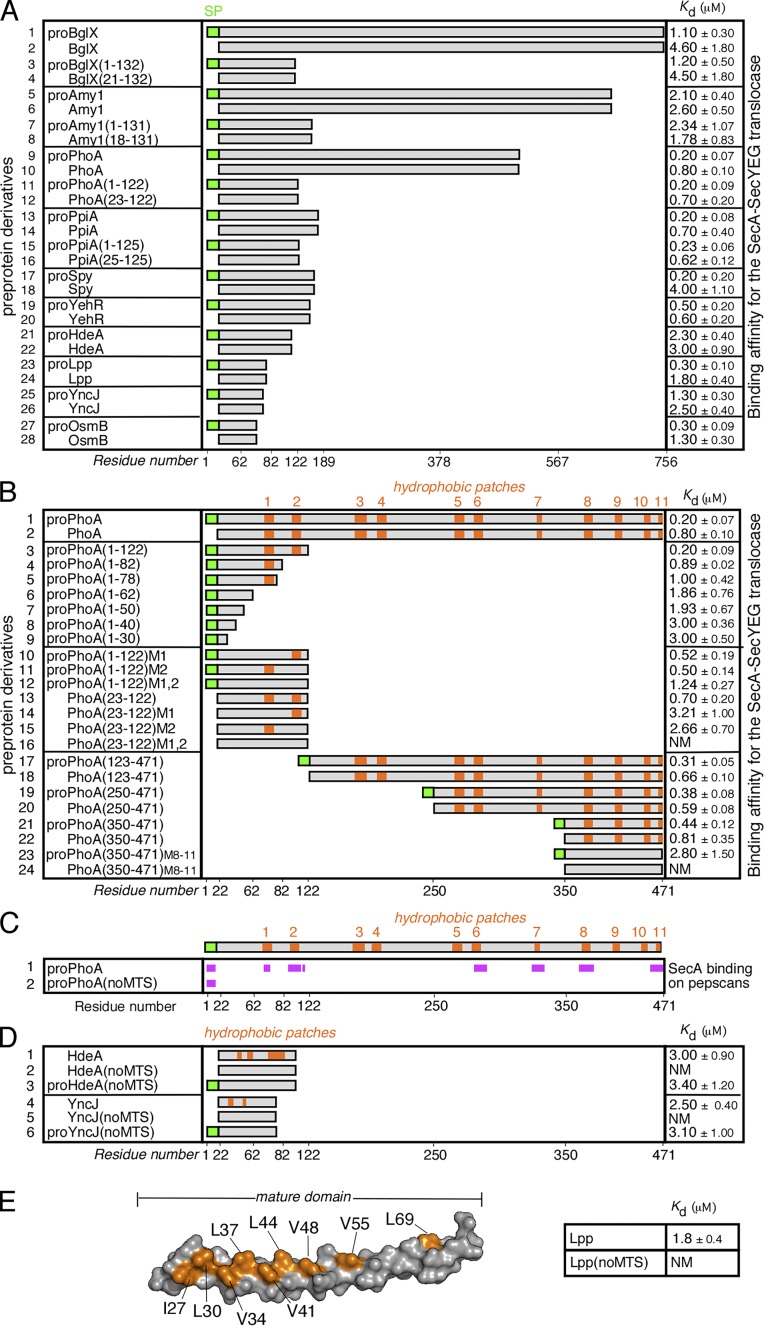
Figure 1.
MTSs in preproteins. (A) Equilibrium dissociation constants (Kd; micromoles; right) of the indicated preproteins, their mature domains and truncated analogues for the wild-type SecA-SecYEG translocase. No detectable binding of proPhoA or derivatives occurs to the SecYEG-inverted membrane vesicles in the absence of SecA (for detailed analysis, see Gouridis et al., 2009). n = 3–9. x axis indicates preprotein length in residues. SP, signal peptide. (B) Hydrophobic patches (HPs; orange) in the mature domain of PhoA (see also Fig. S1 A) and their contribution to targeting. The Kd of the indicated protein derivatives for the translocase were determined; n = 3–9. M1,2 and M8–11, hydrophobicity-reducing mutations in the indicated HPs of PhoA; the mutated residues are detailed in Fig. S1 A. x axis: proPhoA residues. (C) Binding experiments of soluble SecA onto proPhoA or proPhoA(noMTS) peptide arrays are summarized; n = 6 (see also Fig. S2 A). HPs are indicated; x axis: proPhoA residues. (D) HPs (orange) in the secretory proteins HdeA and YncJ (see also Fig. S1 A) and their contribution to targeting. The Kd of the indicated protein derivatives for the translocase were determined; n = 3–6. noMTS, hydrophobicity-reducing mutations in all HPs; the mutated residues are detailed in Fig. S1 A. (E) Left: 3D surface representation of the Lpp structure (PDB: 1EQ7; a single protomer is shown). The residues that were mutated in Lpp(noMTS), shown in orange, are detailed in Fig. S1 B. The Kd of Lpp(noMTS) for the translocase was determined (right); n = 3. NM in C–E, nonmeasurable binding for the translocase (i.e., >20 µM). Affinity values in A, B, D, and E represent means ± SEM.