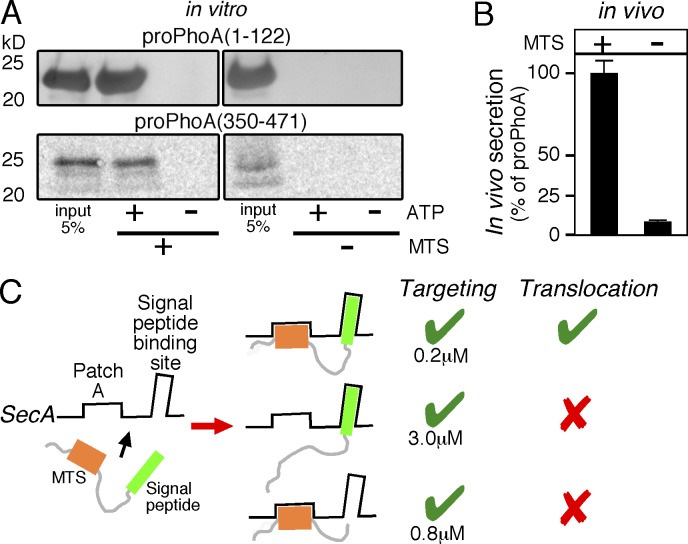
Figure 2.
MTSs are essential for protein secretion. (A) Representative, in vitro, SecA-dependent translocation assays of proPhoA(1–122)M1,2 compared with proPhoA(1–122) (top) and proPhoA(350–471)M8-11 compared with proPhoA(350–471) (bottom) into the lumen of SecYEG containing inverted membrane vesicles; n = 3. 5% of the input is indicated. (B) In vivo secretion of proYncJ-PhoA (left) and proYncJ(noMTS)-PhoA (right) was compared with that of proPhoA (considered as 100%) under identical conditions (MC4100 cells; OD600 = 0.2; 0.002% wt/vol arabinose; 30min; 30°C). In all cases, the measured PhoA enzymatic activity (Gouridis et al., 2009) was normalized to the amount of PhoA or fusion-PhoA protein produced. This provided a means to quantitate the in vivo secretion of all three proteins. The alkaline phosphatase units per microgram PhoA for cells expressing proPhoA was considered 100%; proYncJ-PhoA and proYncJ(noMTS)-PhoA values were expressed as a percentage of this value. n = 5. Values are expressed as means ± SEM. (C) Schematic summary of requirements for preprotein targeting and translocation (as indicated). Preproteins are bivalent ligands with distinct binding sites on SecA. Targeting to the translocase is efficiently achieved by either targeting element, the signal peptide (middle) or one/more MTSs (bottom), independently. However, preprotein translocation requires binding of both (top).