Figure 4.
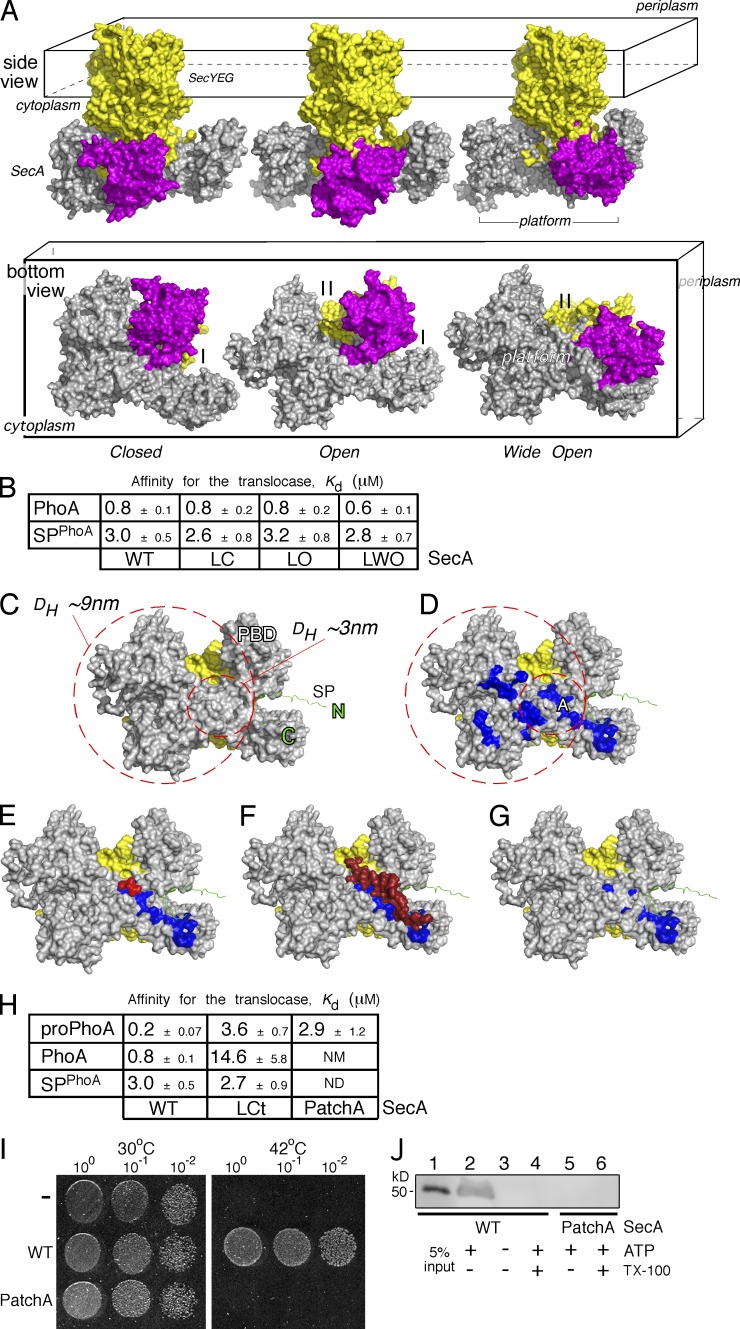
The mature domain–binding site onto SecA. (A) The E. coli SecA (gray)–SeYEG (yellow) was modeled after the Thermotoga maritima translocase in three conformational states, based on PBD (purple) positioning: closed (left), open (middle), and wide open (right). Side and bottom views are shown (as indicated). I, II: SecA clamps. (B) Kd measurements of PhoA and its signal peptide (SPPhoA) for the wild-type (WT), locked closed (LC), locked open (LO), and locked wide open (LWO) SecA bound to SecYEG-inverted membrane vesicles. proPhoA(1–30) was used as SPPhoA. Affinity values represent means ± SEM; n = 3. (C) Potential space occupied by an incoming preprotein onto the cytoplasmic side (platform) of a SecA(gray)–SecYEG(yellow) translocase; signal peptide is in green. The inner circle represents the minimum area a translocation-competent preprotein would occupy, depicted here by the predicted DH of the smallest known preprotein (proEcnA; ∼3 nm; Table S8). The bigger circle represents the area that the expanded, translocation-competent proPhoA would occupy, based on our experimental measurements (∼7 nm; Figs. 3 A and S3 A). (D) Hydrophobic patches (blue; PatchA is indicated) on SecA’s cytoplasmic platform (the rest as in C). (E–G) Structural models of (E) a tripeptide (red; Zimmer and Rapoport, 2009) bound on PatchA (blue) of SecA (detailed interactions in Fig. S5 B) and (F) the C-tail of SecA (dark red) shielding PatchA (blue; Hunt et al., 2002). The C-tail directly interacts with or shields PatchA residues (detailed interactions in Fig. S5 B) but only partially occludes the signal peptide–binding site on SecA (Gelis et al., 2007; see also Fig. S6 E). Signal peptide–binding-determinant residues (i.e., L306; Fig. S6 E) remain exposed and available for interaction at this state. (G) SecA(PatchA) mutant. Four conserved PatchA amino acids (see also Fig. S5 C) were substituted with alanyl residues (M191A/F193A/I224A/I225A) to disrupt the continuum of its hydrophobic surface. (H) Kd measurements of PhoA, proPhoA, and its signal peptide (SPPhoA) for the wild-type, locked C-tail (LCt), and PatchA SecA bound to SecYEG-inverted membrane vesicles; Affinity values represent means ± SEM; n = 3–12. proPhoA(1–30) was used as SPPhoA. NM: nonmeasurable binding (i.e., >20 µM). (I) Representative, in vivo, genetic complementation assay of the E. coli BL21.19(secAts) strain by either an empty vector (−) or wild-type secA or secA(PatchA) mutant; n = 3. Only wild-type secA allows cell growth at 42°C. An identical plate, grown in parallel, at 30°C is shown; the dilutions of cells that were used are indicated. (J) Representative, in vitro SecA-dependent translocation of proPhoA into wt SecYEG-inverted membrane vesicles using wild-type SecA or SecA(PatchA) mutant under the same conditions; n = 3. 5% of the proPhoA input is indicated. Migration of ovalbumin (prestained protein molecular mass marker; Thermo Fisher Scientific).