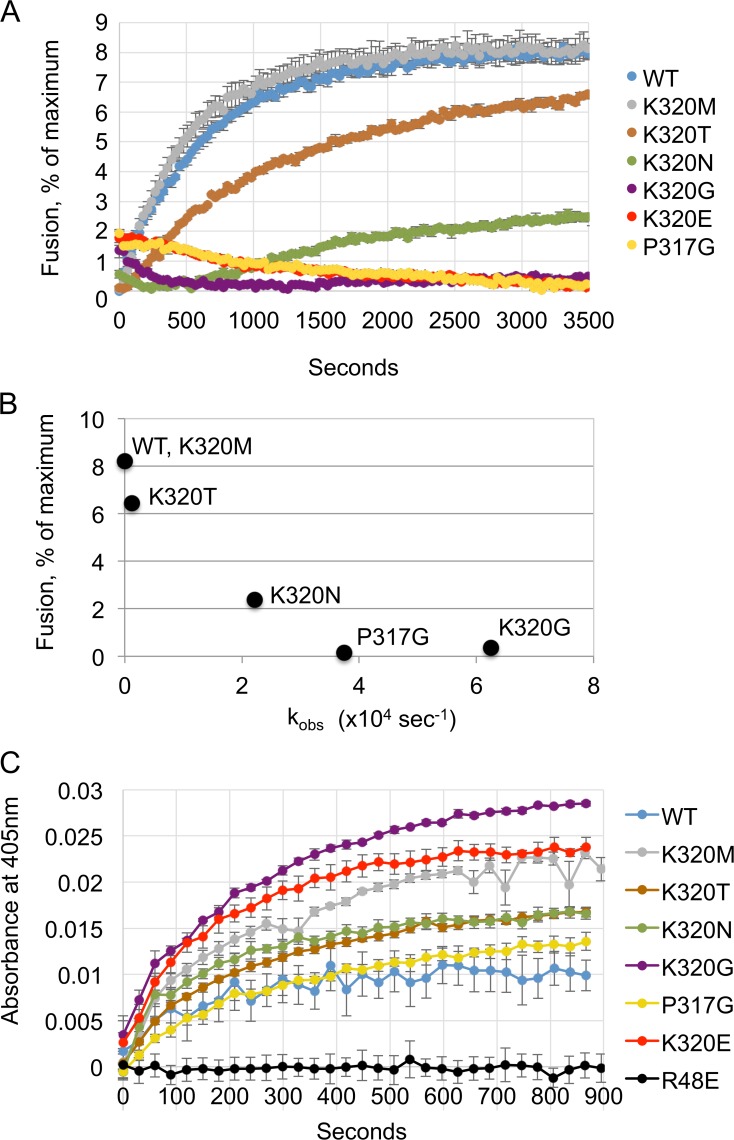
Figure 7.
Crossover dimer stability correlates more closely with fusion than tethering. (A) Mutant variants are variably defective in in vitro fusion activity. The full-length DATL version of each mutant variant was reconstituted into donor and acceptor vesicles at a 1:1,000 protein/lipid ratio. Fusion was monitored as the dequenching of MB-labeled lipid present in the donor vesicles (0.6 mM total lipid) over time after addition of 1 mM GTP (n = 3 replicates; ±SEM). (B) Fusion activity closely parallels crossover dimer stability. The apparent dissociation rate constant for each variant, calculated by fitting the mean of three traces (from Fig. 5 B) to an exponential decay equation (Materials and methods), is plotted against the mean percent fusion (SEM < 0.3%) in vitro (endpoint of A) achieved by the same variant. (C) Vesicle tethering activity does not correlate with crossover dimer stability. The full-length DATL version of each mutant variant was reconstituted into vesicles at a 1:1,000 protein/lipid ratio (0.6 mM total lipid). Tethering by each variant was monitored as the increase in 405-nm absorbance over time after addition of 1 mM GTP (n = 3 replicates; ±SEM). WT, wild type.