Figure 3.
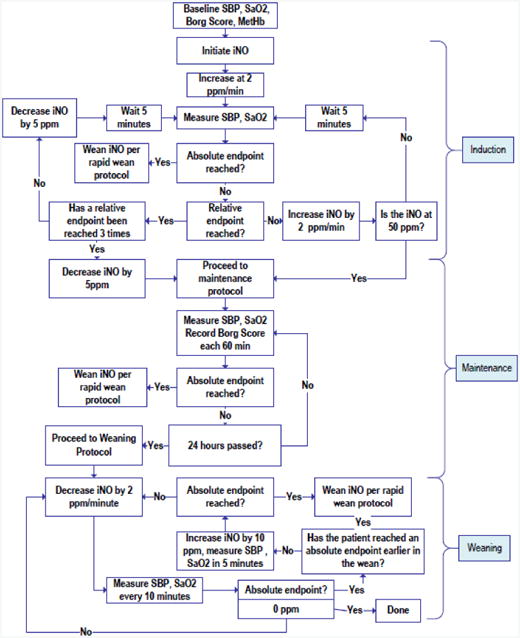
Protocol for administering and weaning study drug. Relative endpoints included persistent symptoms or signs, that in the judgment of the investigator, appeared to be worsened by increasing the inhaled NO ppm and improved by decreasing the inhaled NO ppm. Absolute endpoints included: respiratory distress prompting the clinical care team to plan for mechanical or noninvasive ventilatory support, hypoxemia (SaO2 < 90% for 15 min), circulatory shock (SBP< 90 mm Hg for 15 min in consecutive measurements), new neurologic signs or any ventricular arrhythmias or sustained supraventricular arrhythmia with hypotension or refractory to intravenous medication for more than one hour, ECG changes, MtHb >10% for more than one hour, or persistent vomiting).
The rapid wean protocol was an immediate halving of the inspired concentration and assessment of clinical status; if the patient were improved, the NO was reduced to 0 ppm.
