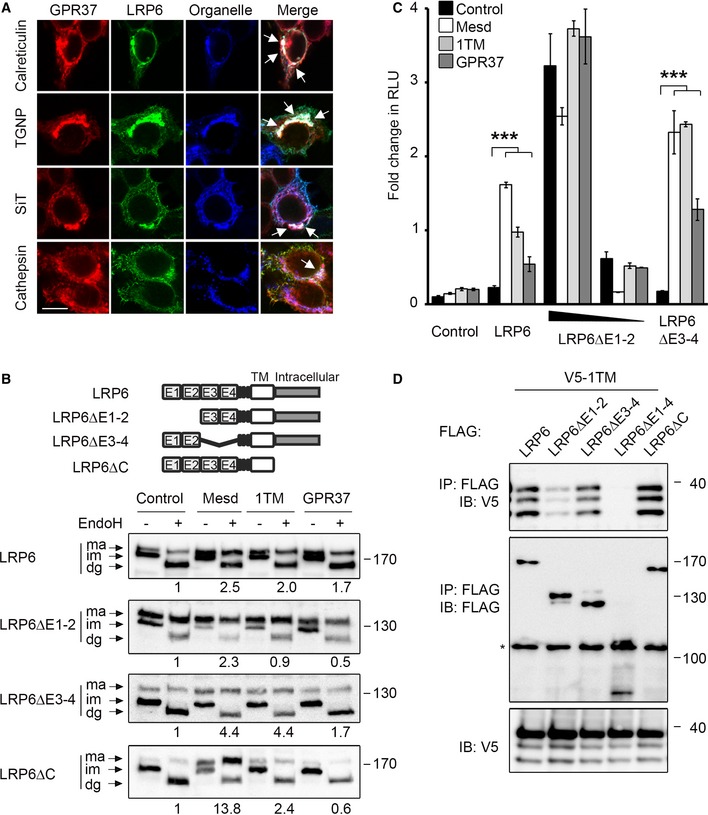
Immunofluorescence microscopy of HEK293T cells transfected with V5‐GPR37 and HA‐LRP6 together with the organelle markers mCherry‐calreticulin (ER), mCherry‐TGNP (Golgi), mCherry‐SiT (trans‐Golgi), or mCherry‐cathepsin (lysosome), which are shown in blue. White signal in merge shows co‐localization of all three labels (arrows). Scale bar: 10 μm.
Representative immunoblots of HEK293T cells transfected with the indicated LRP6 deletion constructs and co‐transfected with control vector, Mesd, GPR37‐1TM, or GPR37. Cell lysates were subjected to EndoH treatment as indicated. Ma, mature LRP6; im, immature; dg, deglycosylated. Numbers under lanes indicate the ratio of mature (ma) to immature (im) LRP6 band from densitometric analysis. Shown are representative experiments that were carried out three times.
Topflash reporter assay in HEK293T cells transfected with the indicated LRP6 constructs and co‐transfected with control vector, Mesd, GPR37‐1TM, or GPR37. Cells were treated as indicated (mean values ± SD, n = 3; ***P < 0.001, one‐way ANOVA followed by Holm–Sidak test).
Co‐immunoprecipitation of GPR37‐1TM with LRP6 deletion constructs. Immunoblots of immunoprecipitates from HEK293T cells transfected with the indicated constructs. The asterisk indicates an unspecific band. Shown are representative experiments that were performed six times.