Figure 1.
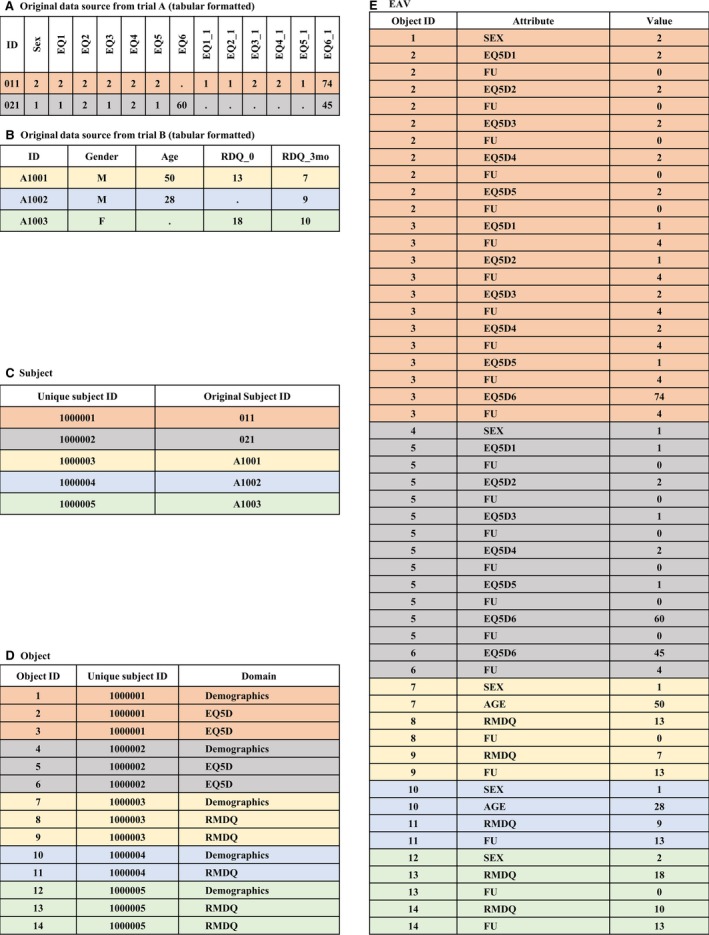
Sample of tabular clinical data in an EAV table. (A) and (B) Examples of original clinical data from two trials in a tabular format, (C) the ‘Subject’ table with a new unique ID for each participant, (D) the ‘Object’ table with an instance of a domain per participant for every derived tabular record, and (E) the ‘EAV’ table with a row for each populated cell and a row for the follow‐up time point where applicable.
