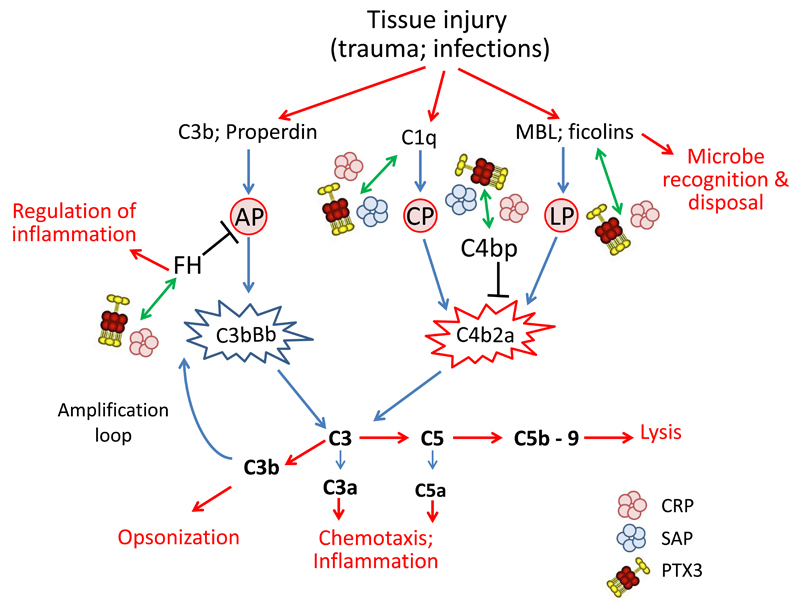
Figure 3. Schematic representation of pentraxins involvement in regulation of complement activity.
PTX3, CRP and SAP have been found to interact with molecules of the complement system. Complement activation is triggered by the interaction of microbes with recognition molecules. The lectin pathway (LP) is activated by interaction of MBL and ficolines with carbohydrates on microbial surface. Specific antibodies can bind to microorganisms forming binding sites for C1q and activate the classical complement pathway (CP). Finally C3 and properdin, once immobilized on a surface such as a microbial cell wall, can activate the alternative pathway (AP). Activation of all the three pathways lead to a cascade of events resulting in opsonization, leukocyte recruitment and cell lysis, activities essential for microbial removal. The first step is the formation of the C3 convertase (C4b2a for CP and LP and C3bBb for AP) that triggers hydrolysis of C3 with formation of C3a, C3b and C5a, and with subsequent formation of C5b-9, the membrane attack complex leading to cell lysis. PTX3, CRP and SAP they all bind C1q, regulating the classical complement pathway. PTX3 and CRP also participate to the regulation of the lectin pathway through interaction with MBL and ficolines. In addition pentraxins can recognize regulators of complement activation, in particular FH, the most important regulator of the alternative pathway, and C4bp, involved in the regulation of the C3 convertase C4b2a, common to the classical and lectin pathways.