Abstract
Islet endothelial cells produce paracrine factors that support β-cell function and growth. Endothelial dysfunction underlies diabetic microvascular complications; thus, we hypothesized that in diabetes, islet endothelial cells become dysfunctional, which may contribute to β-cell secretory dysfunction. Islets/islet endothelial cells were isolated from diabetic B6.BKS(D)-Leprdb/J male (db/db) mice, treated with or without the glucose-lowering agent phlorizin, or from C57BL/6J mice fed a high-fat diet for 18 weeks and appropriate controls. Messenger RNA (mRNA) and/or the protein levels of the cell adhesion molecule E-selectin (Sele), proinflammatory cytokine interleukin-6 (Il6), vasoconstrictor endothelin-1 (Edn1), and endothelial nitric oxide synthase (Nos3; Nos3) were evaluated, along with advanced glycation end product immunoreactivity. Furthermore, an islet endothelial cell line (MS-1) was exposed to diabetic factors (glucose, palmitate, insulin, and tumor necrosis factor-α) for six days. Conditioned media were collected from these cells, incubated with isolated islets, and glucose-stimulated insulin secretion and insulin content were assessed. Islet endothelial cells from db/db mice exhibited increased Sele, Il6, and Edn1 mRNA levels, decreased Nos3 protein, and accumulation of advanced glycation end products. Phlorizin treatment significantly increased Nos3 protein levels but did not alter expression of the other markers. High-fat feeding in C57BL/6J mice resulted in increased islet Sele, Il6, and Edn1 but no change in Nos3. Exposure of islets to conditioned media from MS-1 cells cultured in diabetic conditions resulted in a 50% decrease in glucose-stimulated insulin secretion and 30% decrease in insulin content. These findings demonstrate that, in diabetes, islet endothelial cells show evidence of a dysfunctional phenotype, which may contribute to loss of β-cell function.
The islet contains an extensive capillary network, whose chief component is the islet microvascular endothelial cell (1). Islet endothelial cells have been increasingly recognized as an important source of signals for normal development (2), proliferation (3), and function (4, 5) of the islet β cell. In type 2 diabetes, impaired insulin release from the β cell is a critical element in the pathogenesis of the disease (6, 7). Thus, if islet endothelial cells become dysfunctional under diabetic conditions, that dysfunction could contribute to β-cell failure and, therefore, diabetes progression.
Endothelial dysfunction is characterized by inappropriate increases in cell adhesion, inflammation, and vasoconstriction (8–12) and reduced vasodilation (13, 14). Increased levels of advanced glycation end products (AGEs) also correlate with endothelial dysfunction in diabetic humans (15) and have been shown to result in increased vascular permeability and markers of endothelial dysfunction in animal studies (16, 17). Collectively, these abnormalities underlie diabetic microvascular complications, such as nephropathy, retinopathy, and neuropathy. The severity of these complications is related to the magnitude and duration of hyperglycemia (18, 19). Major intervention studies of both type 1 and 2 diabetes have demonstrated that improving glycemic control reduces microvascular disease (18–20).
Several rodent models of diabetes exhibit altered islet capillary morphology or capillary loss (21–25). However, although one study found biochemical evidence that functional abnormalities in islet endothelial cells can occur independently of capillary loss (26), this is not a widely described phenomenon. In addition, the model used in that study lacked the classic abnormalities seen in type 2 diabetes (i.e., obesity and insulin resistance); thus, the translatability of these findings is unclear. In the present study, we describe a series of in vivo studies examining the effect of diabetes in B6.BKS(D)-Leprdb/J male (db/db) diabetic mice and high-fat feeding in C57BL/6J mice on markers of endothelial function in islets. Furthermore, we performed in vitro studies to examine the ability of diabetes-induced islet endothelial dysfunction to impair insulin release and reduce insulin content in isolated islets.
Materials and Methods
Animals and interventions
Procedures were approved by the VA Puget Sound Health Care System Institutional Animal Care and Use Committee. B6.BKS(D)-Leprdb/J male (db/db) mice (stock no. 697; Jackson Laboratories, Bar Harbor, ME) and nondiabetic male (db/+;+/+) littermates were purchased at 5 weeks of age and studied at 8 or 16 weeks of age (n = 9 to 16). The C57BL/6J background was selected in preference to the naturally occurring C57BL/Ks mice, because the latter exhibits islet degeneration by 16 weeks of age, confounding the ability to isolate islet endothelial cells. A subset of 6-week-old db/db and control mice received the sodium-glucose transporter inhibitor phlorizin [0.8 g/kg/day in propanediol (50% volume-to-volume ratio) intraperitoneally] or vehicle for 2 weeks (n = 5 to 6). This treatment was selected because it lowers glucose via inhibition of glucose reabsorption in the kidney, without having direct effects on either the endothelial cell or islet β cell. C57BL/6J male mice (stock no. 664; Jackson Laboratories) were fed a high-fat diet (60% kcal from fat; D12492; Research Diets, New Brunswick, NJ) or low-fat diet (10% kcal from fat; low-fat diet, D06041501P; Research Diets) for 18 weeks, starting at 10 weeks of age (n = 9 to 10). The body weight was measured and nonfasting blood samples were obtained at the beginning and end of the study, except for the phlorizin study, in which the measurements were taken every 2 to 4 days. At the end of the study, the mice underwent either islet isolation or perfusion fixation (with neutral-buffered formalin) for immunohistochemical analysis of pancreas specimens.
Islet and cell preparation and culture
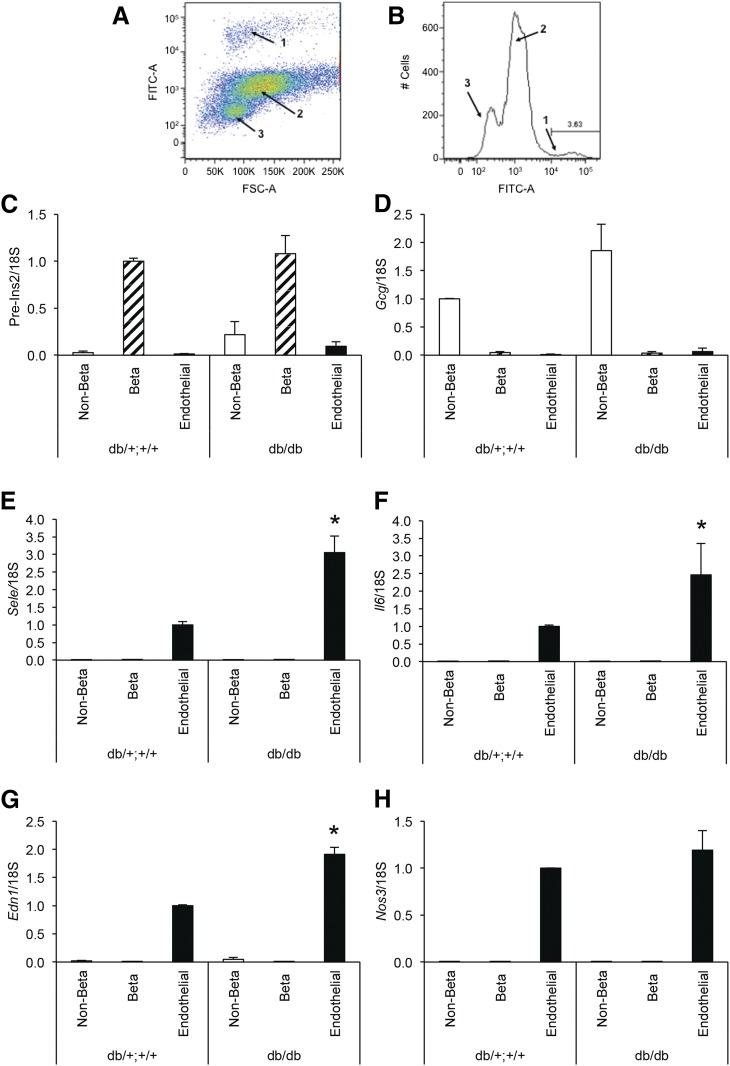
Mouse islets were isolated from mice as described in the previous section or for in vitro studies from C57BL/6J mice (8 to 10 weeks of age) using standard techniques (27). For islet endothelial cell isolation, endothelial cells were labeled intravitally with fluorescein-Lycopersicon esculentum (2 µg/mL; Vector Laboratories, Burlingame, CA) injected intravenously under pentobarbital anesthesia 5 minutes before islet isolation. Islets were then isolated from 3 to 4 mice, pooled, and dissociated (Cell Dissociation Solution; Sigma-Aldrich, St. Louis, MO). Islet cells were sorted on an Aria II high-speed cell sorter (BD Biosciences, Franklin Lakes, NJ), resulting in the separation and collection of islet endothelial cell–, β-cell–, and non-β-cell–enriched populations (28) (Fig. 1A and 1B). Islet cell fractions were harvested for messenger RNA (mRNA) analyses immediately after collection.
Figure 1.
(A, B) Separation of islet cells from 8-week-old db/db and db/+;+/+ mice by fluorescence-activated cell sorting, yielding fluorescently labeled endothelial cells (population 1), β cells (population 2), and non–β-cells (population 3), the latter two differentiated by autofluorescence. (C–H) mRNA levels determined by quantitative polymerase chain reaction in the islet non–β (open bars), β (hatched bars), or endothelial (solid bars) cell populations from db/db or db/+;+/+ littermate controls. Expression of Ins2 pre-mRNA (C) and Gcg mRNA (D) demonstrates good separation among the cellular populations (high in β cells and non–β cells, respectively, and low in the other populations). Endothelial markers Sele (E), Il6 (F), Edn1 (G), and Nos3 mRNA (H) show selective expression in islet endothelial cells. Data are presented as mean ± standard error of the mean; n = 5 per genotype group; *P < 0.05 vs db/+;+/+ endothelial cells.
MS-1 cells (an immortalized line derived from C57BL/6J mouse islet endothelial cells; ATCC, Manassas, VA) were plated (6.5 × 104 cells/mm2) and cultured in RPMI 1640 medium (Life Technologies, Grand Island, NY) containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were treated for 6 days in media containing either 5.5 mM glucose (control) or a combination of glucose (25 mM), sodium palmitate [100 µM, Sigma-Aldrich; complexed to bovine serum albumin at a 5:1 ratio, as described previously (29, 30)], insulin (300 µU/mL; Eli Lilly, Indianapolis, IN), and tumor necrosis factor-α (225 U/mL; ProSpec-Tany TechnoGene Ltd., Ness-Ziona, Israel) for the diabetic conditions. These components were selected to represent the multifactorial diabetic milieu, with concentrations chosen to reflect the levels reported in db/db mice and/or that have been used to study endothelial dysfunction in vitro (23, 31, 32). After the 6-day culture period, all cells were cultured for a further 24 hours in RPMI 1640 medium containing 11.1 mM glucose only, and endothelial cell-conditioned media (CM) were collected during this final 24 hours of culture. The CM glucose levels were then measured and, if necessary, were supplemented back to 11 mM glucose to eliminate any difference in glucose as a variable in the subsequent analyses. C57BL/6J mouse islets were incubated for 48 hours in CM. Islets were also exposed to RPMI 1640 medium not previously exposed to cells or in RPMI 1640 medium with “diabetic” supplements as described (these two conditions served as controls). After this 48-hour culture, glucose-stimulated insulin secretion (GSIS) and insulin content were determined, as previously described (27).
RNA isolation and quantitative polymerase chain reaction
Total RNA was isolated and reverse transcribed as previously described (28). mRNA levels were measured in triplicate using TaqMan Gene Expression assays (Life Technologies) or SyBr green (Eurofins MWG Operon, Huntsville, AL). The specific primers or primer probe sets used are listed in Supplemental Table 1 (50.5KB, docx) . The endogenous controls were 18S ribosomal RNA and cyclophilin. mRNA levels are expressed relative to the appropriate experimental control, using the ΔΔCt method.
Western blotting
Islet protein (50 µg) was separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membrane. Nonspecific binding was blocked (SuperBlock; Thermo Fisher Scientific, Waltham, MA; diluted 1:10 in Tris-buffered saline containing 0.1% [volume-to-volume ratio] Tween-20; pH, 7.4). The membranes were probed with primary antibodies against endothelial nitric oxide synthase (eNOS; 1:1000; ADI-905-386; Enzo Life Sciences, Farmingdale, NY) or the loading control β-actin (1:2000; A2103; Sigma-Aldrich), followed by appropriate peroxidase-conjugated immunoglobulins. Antibody binding was detected using enhanced chemiluminescence (SuperSignal West Femto Chemiluminescent Substrate; Thermo Fisher Scientific). Protein levels are expressed relative to the appropriate experimental control.
Nitric oxide assay
After six days of culture of MS-1 cells in diabetic or control conditions as described in the previous sections, media were removed and the cells incubated for 4 hours in minimal essential media without phenol red (Thermo Fisher Scientific), containing 5.5 mM glucose, 0.1% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. Nitric oxide production was determined by measurement of nitrate, a stable product of nitric oxide in these minimal essential medium samples, using the Nitrate/Nitrite Fluorometric Assay Kit (catalog no. 780051; Cayman Chemical Co., Ann Arbor, MI), according to the manufacturer’s instructions.
Islet morphometry
Formalin-fixed, cryostat pancreas sections (4 µm) were equilibrated in phosphate-buffered saline. Nonspecific binding was blocked (1% [weight-to-volume ratio] bovine serum albumin, 4% normal goat serum [volume-to-volume ratio] in phosphate-buffered saline), and the sections were incubated with primary antibodies against insulin (A0564; 1:100; Dako, Carpinteria, CA), CD31 (550274; 1:25; BD Biosciences, San Jose, CA), and/or AGEs (ab23722; 1:250; Abcam, Cambridge, MA). Primary antibody binding was detected with appropriate Alexa Fluor conjugated immunoglobulins (1:250; Thermo Fisher Scientific), with Hoechst 33258 (2 µg/mL; Sigma-Aldrich) used as a counterstain to visualize cell nuclei.
Islets were identified by insulin immunostaining. Islet-, CD31-, and insulin-positive areas were determined for each islet (average, n = 35 islets analyzed per pancreas specimen). The islet capillary density and β-cell area were computed as follows: ; and , respectively, similar to previously used approaches (27).
Statistical analysis
Data are presented as mean ± standard error of the mean. Differences among experimental groups were identified using Student’s t test, analysis of variance with post hoc analysis, or a nonparametric test (Kruskal-Wallis or Wilcoxon signed rank test) if the data were not normally distributed. P ≤ 0.05 was considered statistically significant.
Results
Markers of endothelial dysfunction in islet endothelial cells
We first demonstrated that E-selectin (Sele), Il6, endothelin-1 (Edn1), and endothelial nitric oxide synthase (Nos3), well-described markers of endothelial cell function (8–14), were selectively expressed in flow cytometry-enriched islet endothelial cells from 8-week-old diabetic db/db mice and littermate db/+;+/+ controls (Fig. 1). Effective separation of islet endothelial, β-cell, and non–β-cell populations by fluorescence-activated cell sorting was verified by mRNA analysis. Ins2 pre-mRNA and Gcg mRNA levels were high in the β- and non–β-cell fractions, respectively, and both were very low in the endothelial cell fractions (Fig. 1C and 1D). Sele, Il6, Edn1, and Nos3 were present in the enriched islet endothelial cell populations for each experimental group but were undetectable (for Sele, Il6, and Nos3; Fig. 1E, 1F, and 1H, respectively) or expressed only at very low levels (for Edn1) in β- or non–β-cell fractions (Fig. 1G). In enriched primary islet endothelial cells from db/db mice, Sele, Il6, and Edn1 mRNA levels were significantly increased (Fig. 1E–G) compared with islet endothelial cells from the db/+;+/+ mice. In contrast, Nos3 mRNA levels did not differ between genotypes (P = 0.6; Fig. 1H).
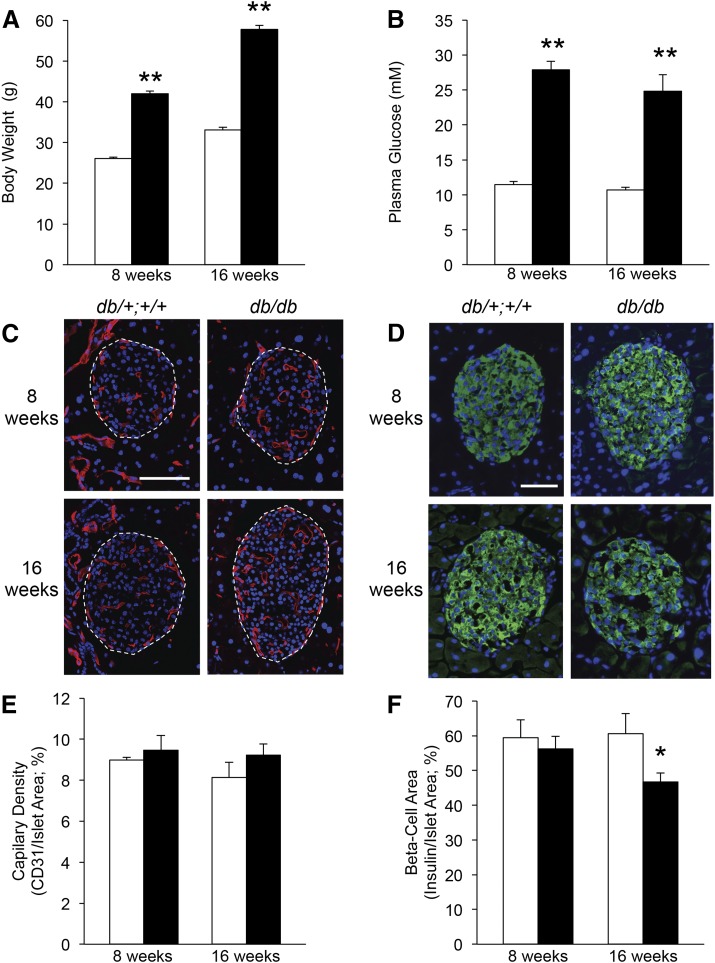
We next determined whether these markers of endothelial dysfunction were detectable in whole islets from db/db diabetic mice and whether this was exacerbated by the duration of diabetes. The body weight and fed glucose levels were already significantly elevated in db/db diabetic mice at 5 weeks of age (27 ± 0.6 g vs 22 ± 0.3 g and 27.6 ± 2.5 mmol/L vs 12.4 ± 0.3 mmol/L for db/db vs db/+;+/+ littermate controls; n = 10 to 16; P < 0.001 for both). This finding of elevated plasma glucose is in contrast to previous reports stating that db/db mice in a C57BL/6J background develop only mild and transient hyperglycemia at 5 weeks of age (33). As expected, the body weight and glucose levels were also elevated in the db/db mice at 8 and 16 weeks of age (Fig. 2A and 2B). Islet capillary morphology was abnormal in the db/db mice at both 8 and 16 weeks of age. The vessels were enlarged or dilated and thickened compared with the db/+;+/+ mice at either age (Fig. 2C). However, no statistically significant difference in islet capillary density was observed between the db/db and db/+;+/+ mice at either age (Fig. 2E). The β-cell area did not differ between the db/db and db/+;+/+ mice at 8 weeks but was significantly reduced at 16 weeks of age (Fig. 2D and 2F).
Figure 2.
Diabetic db/db (solid bars) and nondiabetic littermate db/+;+/+ mice (open bars) were studied at 8 and 16 weeks of age for body weight (A) and nonfasting plasma glucose levels (B). Representative micrographs show islet CD31- (red; C; islet outlines denoted by dashed lines) or insulin immunoreactivity (green; D) and nuclear counterstaining (blue; C, D). Islet capillary density was quantified as the percentage of CD31-positive islet area (E), and β-cell area was quantified as the percentage of insulin-positive islet area (F); n = 10 to 16 for body weight and glucose; n = 4 to 7 for histologic measures; *P < 0.05 and **P < 0.001 vs age-matched db/+;+/+ control. Scale bar = 100 µm (C) and 50 µm (D).
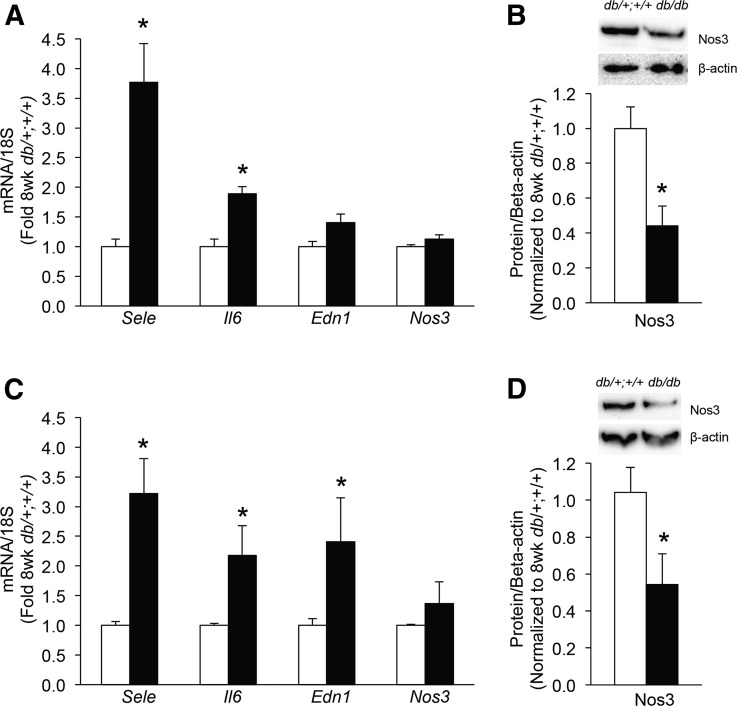
Islet Sele and Il6 mRNA levels were similarly increased in islets from db/db diabetic mice at 8 weeks (Fig. 3A) and 16 weeks (Fig. 3C) of age. Islet Edn1 mRNA was only increased at 16 weeks of age (Fig. 3C). Islet Nos3 mRNA levels did not differ between the db/db and db/+;+/+ mice at either age (Fig. 3A and 3C). However, Nos3 protein levels were significantly decreased in islets from db/db diabetic mice at both 8 and 16 weeks of age (Fig. 3B and 3D).
Figure 3.
Markers of endothelial function in islets from db/db mice (solid bars) or db/+;+/+ littermate mice (open bars) at 8 weeks (A, B) or 16 weeks of age (C, D). mRNA levels are shown for Sele, Il6, Edn1, and Nos3 (A, C) and islet Nos3 protein levels (B, D), with representative western blots for Nos3 and β-actin shown above each bar graph; n = 5 to 9 for islet mRNA levels and n = 3 to 5 for protein levels; *P < 0.05 vs age-matched db/+;+/+ control.
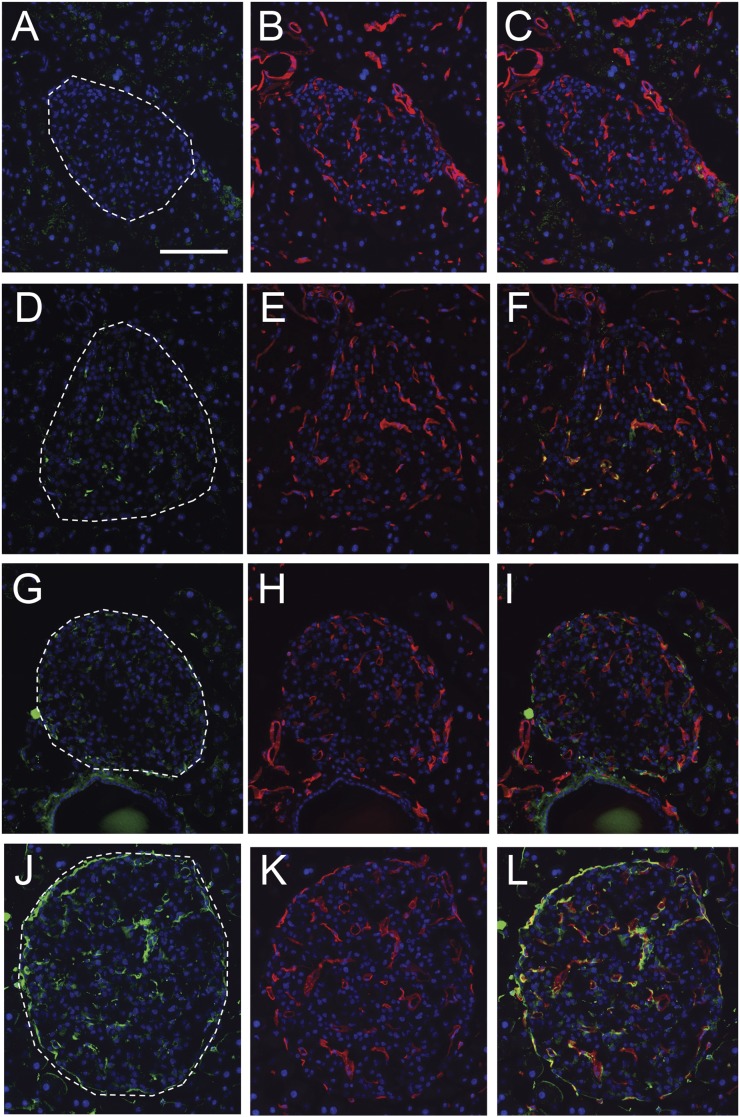
AGE immunoreactivity was present in islets, exclusively within islet endothelial cells in 8-week-old db/db mice (Fig. 4D–F) but was absent from islets in age-matched db/+;+/+ controls (Fig. 4A–C). More extensive AGE immunoreactivity was observed in islets from db/db mice at 16 weeks of age (Fig. 4J–L) and was again present within or closely apposed to islet endothelial cells (Fig. 4L), with little to no staining observed in db/+;+/+ controls at 16 weeks of age (Fig. 4G–I).
Figure 4.
Representative micrographs of pancreas showing immunoreactivity for AGE (green, A, D, G, J) and CD31 to visualize islet endothelial cells (red; B, E, H, K) with nuclear counterstaining (blue; all panels). Merged images showing colocalization between these two markers (orange or yellow staining) or close apposition thereof (C, F, I, and L). AGE immunoreactivity was absent from islets from 8-week-old db/+;+/+ control mice (A–C) and from those of 16-week db/+;+/+ control mice (G–I). In contrast, AGE immunoreactivity was observed exclusively in CD31-positive cells in 8-week-old db/db diabetic mice (G–I). More extensive AGE immunoreactivity was observed in islets from 16-week-old db/db mice (J–L), all of which was localized within or closely associated with CD31-positive cells. Scale bar = 100 µm.
Effect of phlorizin treatment on markers of islet endothelial dysfunction in db/db mice
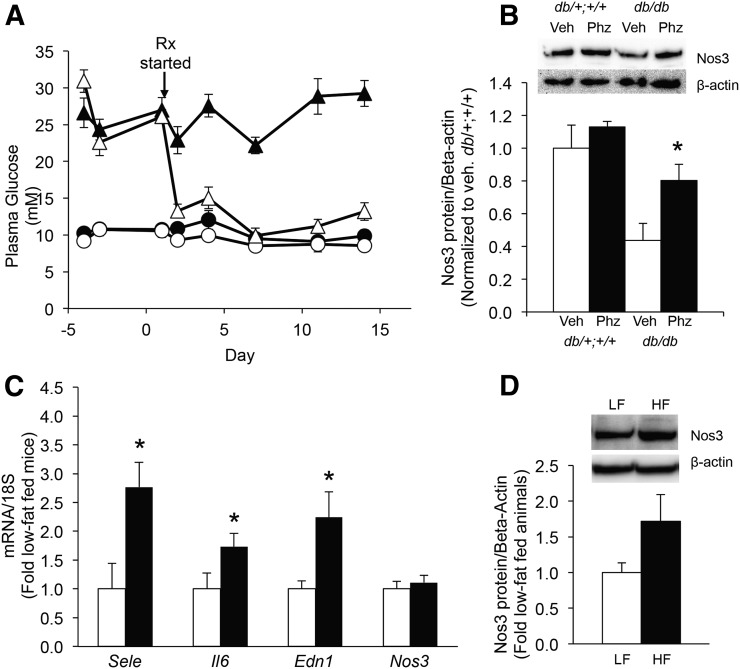
We next determined whether islet endothelial dysfunction occurred secondary to hyperglycemia. Phlorizin treatment normalized blood glucose levels in db/db mice with no effect in the controls (Fig. 5A). Body weight gain was decreased with phlorizin treatment (Supplemental Fig. 1 (34.7MB, tiff) ), although the phlorizin-treated db/db mice still weighed substantially more than the db/+;+/+ mice (vehicle- and phlorizin-treated). Additionally, normalization of plasma glucose levels preceded the onset of decreased weight gain by 3 days. Phlorizin treatment was associated with significant increases in islet Nos3 protein levels (Fig. 5B). No significant changes were seen in Sele, Il6, or Edn1 mRNA levels with phlorizin treatment in db/db vs db/+;+/+ islets (data not shown).
Figure 5.
(A) Nonfasting blood glucose levels in 6- to 8-week-old db/+;+/+ (circles) or db/db (triangles) mice treated with (open symbols) or without (solid symbols) the antihyperglycemic agent phlorizin. (B) Nos3 islet protein levels after the 2-week treatment period with vehicle (Veh)-treated mice shown in open bars and phlorizin (Phz)-treated mice shown in solid bars. Representative western blots showing Nos3 and β-actin protein levels are shown above the bar graph; n = 5 to 6; *P < 0.05 vs all other treatment groups. (C) Islet mRNA levels of endothelial markers in C57BL/6J mice fed a low-fat (open bars) or high-fat (solid bars) diet from 10 to 28 weeks of age; n = 9 to 10 for mRNA; *P ≤ 0.05 vs low-fat control. (D) Islet Nos3 protein in islets from low-fat (open bar) and high-fat (solid bar) fed mice; n = 7. Representative western blots for Nos3 and β-actin protein levels shown above the bar graph.
Islet endothelial dysfunction in high-fat-fed C57BL6/J mice
Next, markers of endothelial dysfunction were quantified in islets from C57BL6/J mice fed a high- (or low-) fat diet for 18 weeks. High-fat feeding resulted in increased nonfasting plasma glucose levels (11.3 ± 0.4 vs 9.6 ± 0.4 mmol/L for high vs low fat, respectively; P < 0.005) and significant weight gain from baseline (25.3 ± 0.4 to 49.0 ± 0.6 g vs 25.4 ± 0.4 to 31.0 ± 1.1 g, respectively; P < 0.001 for final body weight). Significant increases in islet Sele, Il6, and Edn1 mRNA levels were observed in the high-fat-fed mice (Fig. 5C), but Nos3 did not differ at the mRNA or protein level (Fig. 5C and 5D).
Effect of islet endothelial-derived secreted factors on glucose-stimulated insulin release
Finally, we determined whether the secreted factors from islet endothelial cells enhanced insulin release from β cells under normal conditions, and whether this effect was abrogated under conditions that mimic diabetes-induced islet endothelial dysfunction. The 48-hour exposure of C57BL/6J mouse islets to CM generated from MS-1 cells (cultured at 5.5 mM glucose to mimic normal conditions) resulted in significant enhancement of GSIS relative to islets cultured in media not previously exposed to MS-1 cells (0.04 ± 0.007 vs 0.02 ± 0.007 pmol/5 islets/h; n = 3 to 5; P < 0.05), with no effect on basal insulin release (0.006 ± 0.001 vs 0.007 ± 0.003 pmol/5 islets/h). The insulin content tended to increase in islets exposed to CM (14.7 ± 2.4 vs 7.6 ± 1.5 nmol/L/5 islets; P = 0.07).
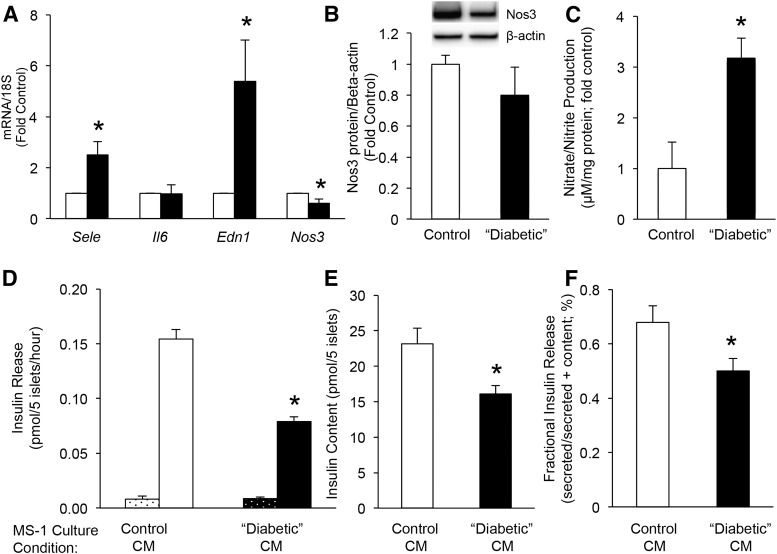
In separate experiments, culture of MS-1 cells for 6 days in diabetic conditions (25 mM glucose, 100 µM palmitate, 300 µU/mL insulin and 225 U/mL tumor necrosis factor-α) followed by a 24-hour washout period in 11.1 mM glucose resulted in increased Sele and Edn1 mRNA levels and reduced Nos3 mRNA levels but no change in Il6 mRNA or Nos3 protein (Fig. 6A and 6B), relative to MS-1 cells cultured in control conditions (5.5 mM glucose). These “diabetic” conditions additionally resulted in increased nitric oxide production (quantified as production of nitrate; Fig. 6C). Exposure of islets to CM from “diabetic” MS-1 cells after the 24-hour washout period resulted in no change in basal insulin release but a 50% reduction in GSIS (Fig. 6D) and a 30% decrease in insulin content (Fig. 6E) compared with islets exposed to CM from MS-1 cells cultured in control conditions. To account for the difference in insulin content between conditions, insulin release was also expressed as fractional insulin release (insulin release/insulin content). This parameter was also significantly decreased in islets exposed to “diabetic” CM (Fig. 6F). Finally, to rule out the possibility that the observed changes in GSIS and insulin content occurred because of the residual presence of the diabetic components used to induce markers of endothelial dysfunction in MS-1 cells, the islets were exposed to this “diabetic” media directly. This resulted in a significant increase in basal insulin release (0.07 ± 0.002 vs 0.01 ± 0.004 pmol/5 islets/h n = 3; P < 0.001), a tendency toward increased GSIS (0.16 ± 0.02 vs 0.12 ± 0.02 pmol/5 islets/h; n = 3; P = 0.1), and a 70% decrease in insulin content (4.0 ± 0.3 vs 13.5 ± 2.1 nmol/L/5 islets; P = 0.01). These changes were markedly different in direction and magnitude from those seen with CM exposure to islets, suggesting that these diabetic components were unlikely to be responsible for the changes in GSIS and insulin content observed in the CM experiments.
Figure 6.
mRNA levels of Sele, Il6, Edn1, and Nos3 (A) in MS-1 cells cultured for 6 days in 5.5 mM glucose (control; open bars) or 25 mM glucose, 100 µmol/L palmitate, 300 µU/mL insulin, and 225 U/mL tumor necrosis factor-α (“diabetic” conditions; solid bars), followed by 24 hours of culture in media containing 11 mM glucose (for both conditions); n = 4; P < 0.05 vs control. (B) Nos3 protein levels in MS-1 cells following the same culture paradigm described for A, with a representative blot for Nos3 and β-actin shown above. (C) Nitrate production from MS-1 cells after 6 days of culture in control or “diabetic” conditions; n = 4; P < 0.05 vs control. (D) Acute basal insulin release (dotted bars) and GSIS (open/solid bars) from islets exposed for 48 hours to CM from MS-1 cells previously cultured in control (open bars) or “diabetic” conditions (solid bars). (E) Insulin content from islets following the same islet culture conditions described for D. (F) Insulin release from the same experiment, expressed as fractional release. Note, before islet exposure, glucose concentrations in CM were matched at 11 mM. (D–F) n = 5; *P < 0.05 vs control CM-exposed islets.
Discussion
It is increasingly recognized that islet endothelial cells produce factors that stimulate β-cell growth and function (3–5). If, under the conditions of diabetes, islet endothelial cells develop a dysfunctional phenotype, they could exhibit altered production of these factors, thereby contributing to islet secretory dysfunction and impaired β-cell growth and survival.
In the present study, we measured the biochemical markers of endothelial dysfunction, as numerous other studies have done (8–14, 16, 17, 32). We found that increased markers of cell adhesion, inflammation, vasoconstriction, and reduced vasodilation and the accumulation of AGEs are present in islet endothelial cells from db/db diabetic mice as early as 8 weeks of age. Except for increases in Edn1 and AGE accumulation, greater diabetes duration did not exacerbate the expression of markers of islet endothelial dysfunction. Furthermore, these markers were specific for islet endothelial cells. Although increased expression of the macrophage marker F4/80 was found in db/db islets, this was detected in the non–β-cell fraction (data not shown) and thus was not coincident with the markers of endothelial dysfunction.
The presence of markers of endothelial dysfunction we observed occurred in the absence of changes in islet capillary density and preceded islet β-cell loss. Thus, the observed islet endothelial cell phenotype is not solely the result of islet capillary loss, the latter having been described in some rodent models of diabetes (21, 24). These findings are more in line with our data from diabetic human islets, in which no deficit in capillary density was observed but thickening and fragmentation of islet capillaries were evident (34). In the present study, we observed enlargement and thickening of islet capillaries, similar to our findings in human diabetes and those reported in several diabetic rodent models (22, 23, 25).
The mechanisms underlying induction of these markers of islet endothelial dysfunction are likely multifactorial. db/db Mice exhibit hyperglycemia, obesity, insulin resistance, and dyslipidemia, any of which could underlie the islet endothelial cell phenotype. In the present study, normalization of glucose levels by phlorizin resulted in an increase in Nos3 protein in db/db islets to levels similar to those of the littermate controls. The levels of Sele, Il6, and Edn1 expression were not changed by 2 weeks of phlorizin treatment; however, it is possible that normalization of glucose levels over a longer period would be effective in reducing these markers. A role for hyperglycemia to decrease Nos3 protein levels in diabetic islets is consistent with the findings of previous studies showing that hyperglycemia impairs endothelial-dependent vasodilation in humans (35) and decreases Nos3 activity and/or production in cultured mouse aortic endothelial cells (36). Furthermore, phlorizin treatment has been shown not to affect lipid levels (31) and did not prevent obesity in db/db mice. The lack of decreased Nos3 levels in islets from high-fat fed C57BL/6J mice in the present study might thus be explained by the absence of severe, chronic hyperglycemia in that model. Taken together, our data suggest that hyperglycemia mediates Nos 3 deficiency but not other aspects of islet endothelial dysfunction.
In contrast, our data from C57BL/6J mice demonstrated that high-fat feeding reproduced the increases in Sele, Il6, and Edn1 seen in db/db diabetic mice. This suggests that features of diabetes such as dyslipidemia might underlie upregulation of these molecules. Increased E-selectin and IL6 have been described in subjects with adverse lipid profiles and vascular disease, in the absence of diabetes (37). Our findings of islet endothelial dysfunction in islets from db/db mice are in line with one previous report showing markers of endothelial activation in islets from diabetic Goto-Kakizaki (GK) rats (26). The GK rat is a lean, relatively insulin-sensitive model of type 2 diabetes. Thus, severe insulin resistance does not appear to be required for the increased expression of islet endothelial dysfunction markers. Finally, the findings from that study, together with our in vitro data showing that diabetic factors can induce some aspects of the islet endothelial phenotype and our in vivo data from the high-fat and phlorizin studies, rule out the presence of the db mutation per se (GK rats and C57BL/6J mice do not harbor the db mutation) as a causative factor in the development of islet endothelial dysfunction.
Islet endothelial cells are now recognized to promote β-cell function and growth (3–5). In line with one other published study (4), our data have demonstrated that factors secreted from islet endothelial cells under normal conditions results in a 50% enhancement of GSIS and tends to increase insulin content. Furthermore, we have now shown that exposure of islets to factors secreted from islet endothelial cells cultured in media that mimic diabetic conditions results in a 50% decrease in GSIS and a 30% reduction in insulin content. The observed changes (increase or decrease) in insulin release occurred in the face of a similar directional change in insulin content. Thus, it is conceivable that the changes in insulin content underlie the observed effects on insulin release. We believe this is unlikely, however, because insulin released in response to glucose stimulation represents only a small fraction of the β-cell’s insulin content (38). Also, the insulin content was not limiting under any of the experimental conditions tested. Direct exposure of islets to the “diabetic” media used for islet endothelial culture resulted in increased insulin release under basal conditions and a tendency toward an increase in GSIS, despite a 70% reduction in insulin content. Finally, for the CM experiments, insulin release was still decreased when the change in insulin content was accounted for (so-called fractional insulin release). Thus, data from the present study support our hypothesis that diabetes-induced islet endothelial dysfunction contributes to impaired insulin release.
Exposure of MS-1 cells to “diabetic” components resulted in upregulation of some, but not all, markers of islet endothelial dysfunction. E-selectin and endothelin-1 were significantly upregulated, as expected. eNOS mRNA was decreased, but the protein levels were not substantially different. We believe this latter finding might have resulted from the duration of the in vitro study, namely that decreased eNOS protein might only occur with long-term hyperglycemia, an observation that would be in line with our in vivo findings that eNOS protein is decreased in islets from db/db mice but not those fed a high-fat diet. In addition, increased nitric oxide production was observed, consistent with activation of inflammatory stress pathways in the islet endothelial cell and in line with a previous study showing activation of inducible NOS-mediated nitric oxide production in primary islet endothelial cells exposed to cytokines (39). Unexpectedly, IL-6 was not increased in this model. The reason for this is unclear, although exposure of MS-1 cells to high glucose alone for six days did result in a 40% increase in IL-6 expression (data not shown), suggesting that other components of the “diabetic” cocktail could have suppressed IL-6 production, resulting in no net change.
The observed increase in endothelin-1 in islet endothelial cells exposed to “diabetic” media could have directly modulated insulin release, as proposed previously (40); however, endothelin-1 would be expected to enhance insulin release, which is in the opposite direction to explain the findings in the present study. Rather, we propose that the increased endothelin-1 and E-selectin measured in the present study will be useful in uncovering a dysfunctional phenotype in the islet endothelial cell; however, other endothelial-derived molecules are likely to be important in modulating insulin release. For example, islet endothelial cells produce multiple extracellular matrix components, which engage β cells, and elicit downstream signaling. In particular, activation of extracellular matrix molecule receptors such as integrins (41) or E-cadherin results in improved insulin release and β-cell survival. Thus, islet endothelial dysfunction could result in changes in islet extracellular matrix production and/or composition, which has been described in human type 2 diabetes and models thereof, which could, in turn, result in decreased insulin release, insulin content, and/or β-cell survival.
In conclusion, markers of islet endothelial inflammation and impaired vasoactivity are present in islets from diabetic db/db mice, and this dysfunctional islet endothelial phenotype might represent an unrecognized mechanism that contributes to decreased β-cell function in type 2 diabetes.
Acknowledgments
We thank Phillip Bergquist, Breanne Barrow, Christina Braddock, Maria Cone, Daryl Hackney, Atiqur Rahman, and Jessica Wilkins-Gutierrez for technical support and the metabolism group at the Veterans Affairs Puget Sound Health Care System in Seattle for helpful discussions during the performance of this work.
Acknowledgments
This work was supported by the Department of Veterans Affairs, Veterans Affairs Puget Sound Health Care System (Seattle, WA), National Institutes of Health grants R01 DK088082 (R.L.H.) and P30 DK017047 (University of Washington Diabetes Research Center), and a University of Washington Royalty Research Fund Award. A.W.L. was supported by the HHMI Medical Research Fellows Program and the National Institute of Diabetes and Digestive and Kidney Diseases Medical Research Program in Diabetes through grant T32 DK007247. M.F.H. was supported by grants T32 HL007028 and F32 DK109584. Z.R., A.O., and E.C.B. were supported by the University of Washington Medical Student Research Training Program and the National Institute of Diabetes and Digestive and Kidney Diseases Medical Research Program in Diabetes through T32 DK007247.
Disclosure Summary: The authors have nothing to disclose
Footnotes
- AGE
- advanced glycation end product
- CM
- endothelial cell-conditioned medium
- db/+;+/+
- nondiabetic male
- db/db
- B6.BKS(D)-Leprdb/J
- Edn1
- endothelin-1
- eNOS
- endothelial nitric oxide synthase
- GK
- Goto-Kakizaki
- GSIS
- glucose-stimulated insulin secretion
- Il6
- interleukin-6
- mRNA
- messenger RNA
- Nos3
- endothelial nitric oxide synthase
- Sele
- E-selectin
References
- 1.Bonner-Weir S, Orci L. New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes. 1982;31(10):883–889. [DOI] [PubMed] [Google Scholar]
- 2.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294(5542):564–567. [DOI] [PubMed] [Google Scholar]
- 3.Johansson M, Mattsson G, Andersson A, Jansson L, Carlsson PO. Islet endothelial cells and pancreatic beta-cell proliferation: studies in vitro and during pregnancy in adult rats. Endocrinology. 2006;147(5):2315–2324. [DOI] [PubMed] [Google Scholar]
- 4.Johansson A, Lau J, Sandberg M, Borg LA, Magnusson PU, Carlsson PO. Endothelial cell signalling supports pancreatic beta cell function in the rat. Diabetologia. 2009;52(11):2385–2394. [DOI] [PubMed] [Google Scholar]
- 5.Olerud J, Mokhtari D, Johansson M, Christoffersson G, Lawler J, Welsh N, Carlsson PO. Thrombospondin-1: an islet endothelial cell signal of importance for β-cell function. Diabetes. 2011;60(7):1946–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahn SE. Clinical review 135: The importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab. 2001;86(9):4047–4058. [DOI] [PubMed] [Google Scholar]
- 7.Porte D., Jr Banting lecture 1990: beta-cells in type II diabetes mellitus. Diabetes. 1991;40(2):166–180. [DOI] [PubMed] [Google Scholar]
- 8.Meigs JB, Hu FB, Rifai N, Manson JE. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA. 2004;291(16):1978–1986. [DOI] [PubMed] [Google Scholar]
- 9.De Mattia G, Bravi MC, Laurenti O, Moretti A, Cipriani R, Gatti A, Mandosi E, Morano S. Endothelial dysfunction and oxidative stress in type 1 and type 2 diabetic patients without clinical macrovascular complications. Diabetes Res Clin Pract. 2008;79(2):337–342. [DOI] [PubMed] [Google Scholar]
- 10.Weis M, Wildhirt SM, Schulze C, Rieder G, Wilbert-Lampen U, Wolf WP, Arendt RM, Enders G, Meiser BM, von Scheidt W. Endothelin in coronary endothelial dysfunction early after human heart transplantation. J Heart Lung Transplant. 1999;18(11):1071–1079. [DOI] [PubMed] [Google Scholar]
- 11.Barton M, Haudenschild CC, d’Uscio LV, Shaw S, Münter K, Lüscher TF. Endothelin ETA receptor blockade restores NO-mediated endothelial function and inhibits atherosclerosis in apolipoprotein E-deficient mice. Proc Natl Acad Sci USA. 1998;95(24):14367–14372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piconi L, Quagliaro L, Da Ros R, Assaloni R, Giugliano D, Esposito K, Szabó C, Ceriello A. Intermittent high glucose enhances ICAM-1, VCAM-1, E-selectin and interleukin-6 expression in human umbilical endothelial cells in culture: the role of poly(ADP-ribose) polymerase. J Thromb Haemost. 2004;2(8):1453–1459. [DOI] [PubMed] [Google Scholar]
- 13.Veves A, Akbari CM, Primavera J, Donaghue VM, Zacharoulis D, Chrzan JS, DeGirolami U, LoGerfo FW, Freeman R. Endothelial dysfunction and the expression of endothelial nitric oxide synthetase in diabetic neuropathy, vascular disease, and foot ulceration. Diabetes. 1998;47(3):457–463. [DOI] [PubMed] [Google Scholar]
- 14.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377(6546):239–242. [DOI] [PubMed] [Google Scholar]
- 15.Tan KC, Chow WS, Ai VH, Metz C, Bucala R, Lam KS. Advanced glycation end products and endothelial dysfunction in type 2 diabetes. Diabetes Care. 2002;25(6):1055–1059. [DOI] [PubMed] [Google Scholar]
- 16.Wautier JL, Zoukourian C, Chappey O, Wautier MP, Guillausseau PJ, Cao R, Hori O, Stern D, Schmidt AM. Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy: soluble receptor for advanced glycation end products blocks hyperpermeability in diabetic rats. J Clin Invest. 1996;97(1):238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baumgartner-Parzer SM. Glycemia and regulation of endothelial adhesion molecules. Horm Metab Res. 1997;29(12):636–638. [DOI] [PubMed] [Google Scholar]
- 18.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. [DOI] [PubMed] [Google Scholar]
- 19.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 20.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F; ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura M, Kitamura H, Konishi S, Nishimura M, Ono J, Ina K, Shimada T, Takaki R. The endocrine pancreas of spontaneously diabetic db/db mice: microangiopathy as revealed by transmission electron microscopy. Diabetes Res Clin Pract. 1995;30(2):89–100. [DOI] [PubMed] [Google Scholar]
- 22.Mizuno A, Noma Y, Kuwajima M, Murakami T, Zhu M, Shima K. Changes in islet capillary angioarchitecture coincide with impaired B-cell function but not with insulin resistance in male Otsuka-Long-Evans-Tokushima fatty rats: dimorphism of the diabetic phenotype at an advanced age. Metabolism. 1999;48(4):477–483. [DOI] [PubMed] [Google Scholar]
- 23.Shao J, Iwashita N, Ikeda F, Ogihara T, Uchida T, Shimizu T, Uchino H, Hirose T, Kawamori R, Watada H. Beneficial effects of candesartan, an angiotensin II type 1 receptor blocker, on beta-cell function and morphology in db/db mice. Biochem Biophys Res Commun. 2006;344(4):1224–1233. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Zhang L, Meshinchi S, Dias-Leme C, Raffin D, Johnson JD, Treutelaar MK, Burant CF. Islet microvasculature in islet hyperplasia and failure in a model of type 2 diabetes. Diabetes. 2006;55(11):2965–2973. [DOI] [PubMed] [Google Scholar]
- 25.Masuyama T, Komeda K, Hara A, Noda M, Shinohara M, Oikawa T, Kanazawa Y, Taniguchi K. Chronological characterization of diabetes development in male spontaneously diabetic Torii rats. Biochem Biophys Res Commun. 2004;314(3):870–877. [DOI] [PubMed] [Google Scholar]
- 26.Lacraz G, Giroix M-H, Kassis N, Coulaud J, Galinier A, Noll C, Cornut M, Schmidlin F, Paul J-L, Janel N, Irminger J-C, Kergoat M, Portha B, Donath MY, Ehses JA, Homo-Delarche F. Islet endothelial activation and oxidative stress gene expression is reduced by IL-1Ra treatment in the type 2 diabetic GK rat. PLoS One. 2009;4(9):e6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hull RL, Shen ZP, Watts MR, Kodama K, Carr DB, Utzschneider KM, Zraika S, Wang F, Kahn SE. Long-term treatment with rosiglitazone and metformin reduces the extent of, but does not prevent, islet amyloid deposition in mice expressing the gene for human islet amyloid polypeptide. Diabetes. 2005;54(7):2235–2244. [DOI] [PubMed] [Google Scholar]
- 28.Hull RL, Johnson PY, Braun KR, Day AJ, Wight TN. Hyaluronan and hyaluronan binding proteins are normal components of mouse pancreatic islets and are differentially expressed by islet endocrine cell types. J Histochem Cytochem. 2012;60:749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Briaud I, Harmon JS, Kelpe CL, Segu VB, Poitout V. Lipotoxicity of the pancreatic beta-cell is associated with glucose-dependent esterification of fatty acids into neutral lipids. Diabetes. 2001;50(2):315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zraika S, Koh DS, Barrow BM, Lu B, Kahn SE, Andrikopoulos S. Neprilysin deficiency protects against fat-induced insulin secretory dysfunction by maintaining calcium influx. Diabetes. 2013;62(5):1593–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kjørholt C, Akerfeldt MC, Biden TJ, Laybutt DR. Chronic hyperglycemia, independent of plasma lipid levels, is sufficient for the loss of beta-cell differentiation and secretory function in the db/db mouse model of diabetes. Diabetes. 2005;54(9):2755–2763. [DOI] [PubMed] [Google Scholar]
- 32.Jin HM, Liu QH, Cao X, Wu ZH, Zhang GP, Zhang M, Sha ZY. Dysfunction of microvascular endothelial cells induced by tumor necrosis factor (TNFalpha): cellular and molecular mechanism. Clin Hemorheol Microcirc. 2000;23(2-4):109–112. [PubMed] [Google Scholar]
- 33.Hummel KP, Coleman DL, Lane PW. The influence of genetic background on expression of mutations at the diabetes locus in the mouse. I. C57BL-KsJ and C57BL-6J strains. Biochem Genet. 1972;7(1):1–13. [DOI] [PubMed] [Google Scholar]
- 34.Brissova M, Shostak A, Fligner CL, Revetta FL, Washington MK, Powers AC, Hull RL. Human islets have fewer blood vessels than mouse islets and the density of islet vascular structures is increased in type 2 diabetes. J Histochem Cytochem 2015:63(8):637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams SB, Goldfine AB, Timimi FK, Ting HH, Roddy MA, Simonson DC, Creager MA. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998;97(17):1695–1701. [DOI] [PubMed] [Google Scholar]
- 36.Srinivasan S, Hatley ME, Bolick DT, Palmer LA, Edelstein D, Brownlee M, Hedrick CC. Hyperglycaemia-induced superoxide production decreases eNOS expression via AP-1 activation in aortic endothelial cells. Diabetologia. 2004;47(10):1727–1734. [DOI] [PubMed] [Google Scholar]
- 37.Miller TI, Borkowsky W, DiMeglio LA, Dooley L, Geffner ME, Hazra R, McFarland EJ, Mendez AJ, Patel K, Siberry GK, Van Dyke RB, Worrell CJ, Jacobson DL, Shearer W, Cooper N, Harris L, Purswani M, Baig M, Cintron A, Puga A, Navarro S, Patton D, Burchett S, Karthas N, Kammerer B, Yogev R, Malee K, Hunter S, Cagwin E, Wiznia A, Burey M, Nozyce M, Chen J, Gobs E, Grant M, Knapp K, Allison K, Garvie P, Acevedo-Flores M, Rios H, Olivera V, Silio M, Borne C, Sirois P, Spector S, Norris K, Nichols S, McFarland E, Barr E, Chambers C, Watson D, Messenger N, Belanger R, Dieudonne A, Bettica L, Adubato S, Scott G, Himic L, Willen E, Willen E; Pediatric HIV/AIDS Cohort Study (PHACS) . Metabolic abnormalities and viral replication are associated with biomarkers of vascular dysfunction in HIV-infected children. HIV Med. 2012;13(5):264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henquin JC, Dufrane D, Nenquin M. Nutrient control of insulin secretion in isolated normal human islets. Diabetes. 2006;55(12):3470–3477. [DOI] [PubMed] [Google Scholar]
- 39.Suschek C, Fehsel K, Kröncke KD, Sommer A, Kolb-Bachofen V. Primary cultures of rat islet capillary endothelial cells: constitutive and cytokine-inducible macrophagelike nitric oxide synthases are expressed and activities regulated by glucose concentration. Am J Pathol. 1994;145(3):685–695. [PMC free article] [PubMed] [Google Scholar]
- 40.Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, Eppler E, Bouzakri K, Wueest S, Muller YD, Hansen AM, Reinecke M, Konrad D, Gassmann M, Reimann F, Halban PA, Gromada J, Drucker DJ, Gribble FM, Ehses JA, Donath MY. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011;17(11):1481–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bosco D, Meda P, Halban PA, Rouiller DG. Importance of cell-matrix interactions in rat islet beta-cell secretion in vitro: role of alpha6beta1 integrin. Diabetes. 2000;49(2):233–243. [DOI] [PubMed] [Google Scholar]