Abstract
Background:
Nonalcoholic fatty liver disease (NAFLD) can be diagnosed early by noninvasive ultrasonography; however, the cost-effectiveness of ultrasonography screening with intensive weight reduction program in metabolic syndrome patients is not clear. This study aims to estimate economic and clinical outcomes of ultrasonography in Thailand.
Methods:
Cost-effectiveness analysis used decision tree and Markov models to estimate lifetime costs and health benefits from societal perspective, based on a cohort of 509 metabolic syndrome patients in Thailand. Data were obtained from published literatures and Thai database. Results were reported as incremental cost-effectiveness ratios (ICERs) in 2014 US dollars (USD) per quality-adjusted life year (QALY) gained with discount rate of 3%. Sensitivity analyses were performed to assess the influence of parameter uncertainty on the results.
Results:
The ICER of ultrasonography screening of 50-year-old metabolic syndrome patients with intensive weight reduction program was 958 USD/QALY gained when compared with no screening. The probability of being cost-effective was 67% using willingness-to-pay threshold in Thailand (4848 USD/QALY gained). Screening before 45 years was cost saving while screening at 45 to 64 years was cost-effective.
Conclusions:
For patients with metabolic syndromes, ultrasonography screening for NAFLD with intensive weight reduction program is a cost-effective program in Thailand. Study can be used as part of evidence-informed decision making.
Translational Impacts:
Findings could contribute to changes of NAFLD diagnosis practice in settings where economic evidence is used as part of decision-making process. Furthermore, study design, model structure, and input parameters could also be used for future research addressing similar questions.
Keywords: cost-effectiveness analysis, metabolic syndrome, nonalcoholic fatty liver disease, ultrasonography screening
Key Points
What is current knowledge?
Liver biopsy is the gold standard for diagnosis of NAFLD despite its invasiveness.
Ultrasonography is widely accepted as a tool to detect moderate to severe NAFLD.
What is new here?
Ultrasonography screening for NAFLD with intensive weight reduction program in metabolic-syndrome patients is cost-effective.
This program could slow down the progression to cirrhosis and hepatocellular carcinoma.
Translational impact
Cost-effectiveness finding of the program could contribute to changes of NAFLD diagnosis practice.
Study design, model structure, and input parameters could be used for similar future research.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) has been recognized as an emerging chronic liver disease worldwide. The prevalence is rising globally aligned with the increase of type 2 diabetes, obesity, and metabolic syndrome. It is around 20% in the general population while it reaches 70% in high-risk groups such as obesity and type 2 diabetes.[1,2] In Thailand, the prevalence of NAFLD in metabolic syndrome had increased up to 67% even in the nonobese patients.[3] NAFLD can progress to liver cirrhosis, hepatocellular carcinoma (HCC), and death and it has been associated with increased mortality.[4–7] Its major causes of death were cardiovascular disease (28%), extrahepatic cancers (25%), and liver-related death (13%). Even if NAFLD represents an important public health burden, the natural history, predictors, and factors determining severity are insufficiently understood because most patients are asymptomatic and underdiagnosed until late complications occur. In metabolic syndrome patients who had early detection of NAFLD, there were several effective interventions to delay the progression such as intensive life style modification, tight control of the risk factors, and specific medications[8] whereas the patients who had late detection will face with the serious and high-cost complications.
Currently, liver biopsy is the gold standard for diagnosis and staging of NAFLD but it cannot be applied to population-based studies because of its highly invasiveness. A recent meta-analysis study stated that ultrasonography had high reliability for detecting moderate to severe NAFLD, with the area under the summary receiving operating characteristics (ROC) curve of.93 when compared with liver histology.[9] The American Association for the Study of Liver Diseases (AASLD) guideline 2012[10] advised against screening for NAFLD in high-risk groups (diabetes or obesity) due to the lack of clear long-term benefits and cost-effectiveness of screening.
The World Health Assembly 2014 recognized that Health Technology Assessment (HTA) of healthcare interventions is a crucial tool that can be used for evidence-informed policy decision making to ensure sustainable healthcare financing under universal health coverage.[11] HTA has been widely used for health benefit package design of universal coverage insurance scheme in many countries including Thailand. At present, ultrasonography screening for NAFLD has not been included in benefit package in Thailand. There have been no studies evaluating cost-effectiveness of ultrasonography screening for NAFLD in global and Thai literature. Thus, this study aimed to evaluate the cost-effectiveness of ultrasonography screening for NAFLD followed with intensive weight reduction program in patients with metabolic syndrome in low to middle income country using Thailand as an example.
2. Methods
2.1. Overall description
A cost-effectiveness analysis was undertaken to estimate relevant costs and health outcomes of ultrasonography screening for NAFLD with intensive weight reduction program in metabolic syndrome patients, compared with usual care, defined as no screening. Intervention of interest is ultrasonography and intensive weight reduction program provided to patients diagnosed as NAFLD. Intensive weight reduction program was chosen because of its clear evidence demonstrating hepatic fibrosis regression.[12] Since long-term outcomes of NAFLD included cirrhosis and HCC, the lifetime time horizon was chosen in this study. We undertook this study using a societal perspective as recommended by the Thailand's HTA guideline.[13] Our findings were presented by incremental cost-effectiveness ratios (ICERs) in US dollars (USD) per quality-adjusted life year (QALY) gained. The interpretation of cost-effectiveness of the findings was based on an official willingness-to-pay (WTP) of 160,000 THB/QALY (4848 USD/QALY) adopted by Thai Health Economic Working Group.[14] The discount rate of 3% was used for both costs and health outcomes.
2.2. Economic model
A hypothetical cohort of 100,000 individuals with metabolic syndrome was simulated in the model. A decision tree was constructed to divide patients into 2 groups; screening with intensive weight reduction program and no screening groups. However, the whole effect of screening with weight reduction such as death and long-term effects cannot be captured with only decision tree model. Therefore, Markov models were developed with a 1-year cycle length to capture long-term costs and health outcomes. In Markov models, patients could remain in the same state or move to other states. No advanced fibrosis (NAF) and indeterminate fibrosis states could progress to advanced fibrosis (AF). Conversely, AF could regress to NAF. Moreover, AF could develop to either compensated or decompensated cirrhosis. Compensated cirrhosis could progress to decompensated cirrhosis. Finally, both compensated and decompensated cirrhosis could transform to HCC and death. Patients in the compensated, decompensated cirrhosis state, and HCC cannot reverse to a primary state as shown in Fig. 1.
Figure 1.
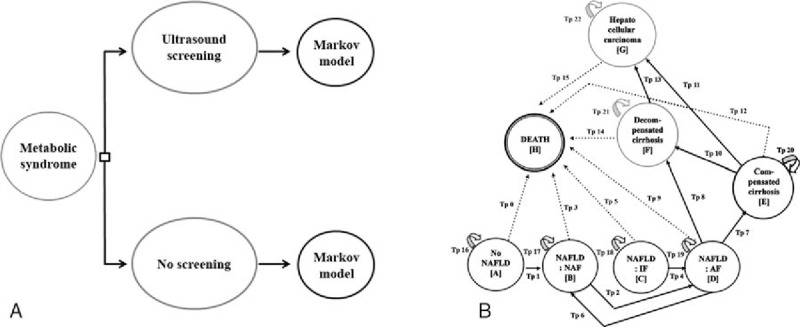
Decision tree and Markov models. A decision tree was constructed to divide patients into 2 groups; screening with intensive weight reduction program and no screening groups. In Markov models, patients could remain in the same state or move to other states.
2.3. Input parameters
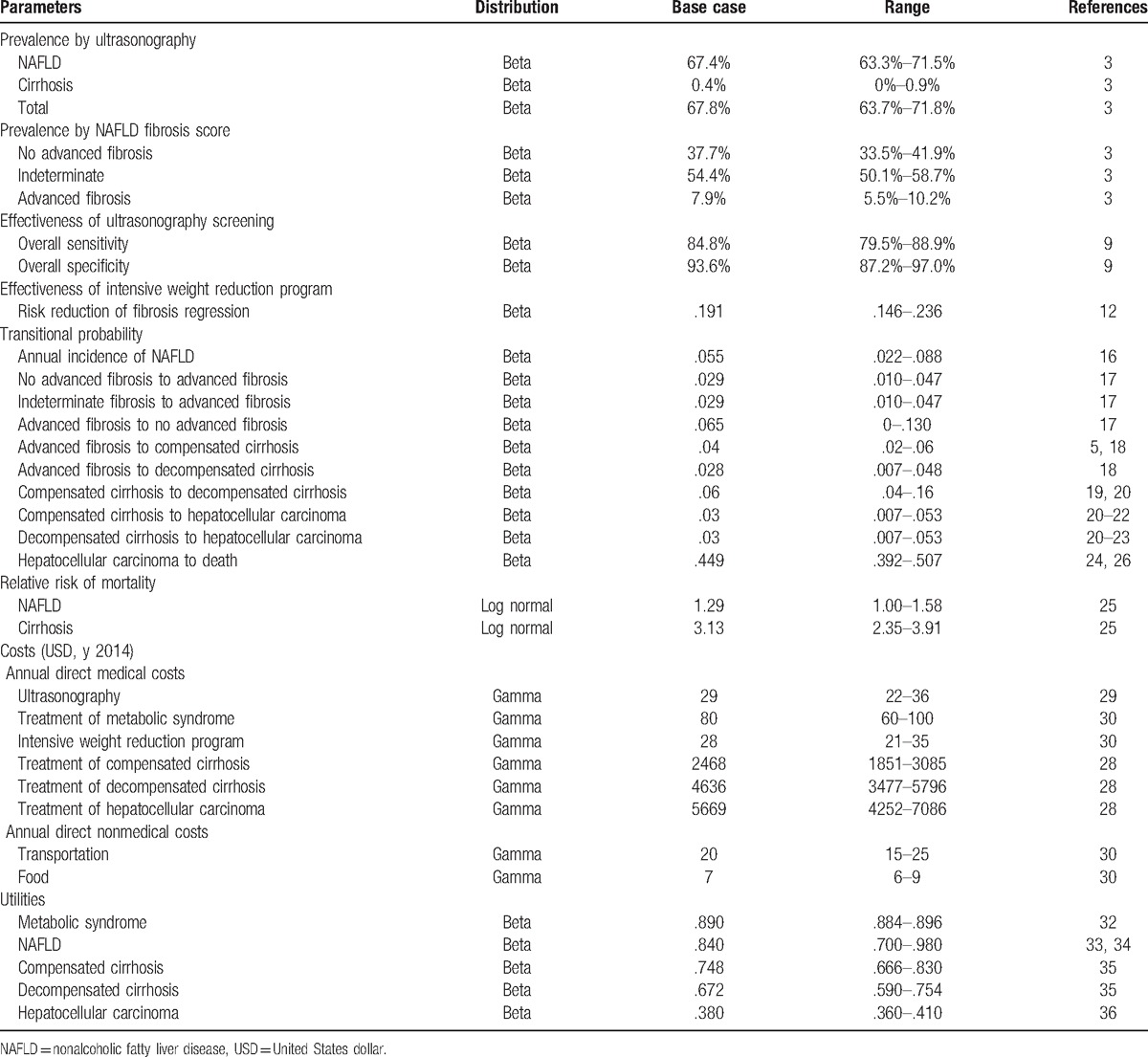
The prevalence of NAFLD and distribution of NAFLD Fibrosis Score were based on a cohort of 509 metabolic syndrome patients according to “the Harmonizing the Metabolic Syndrome definition”[15] recruited from Siriraj Hospital, Mahidol University, a 2111-bed hospital in Bangkok, Thailand.[3] This cohort included patients followed up between November 2011 and October 2013 with the mean age of 61 years. This study was approved by Siriraj Institutional Review Board. All participants received information, and signed an inform consent sheet before participation. Other input parameters (i.e., sensitivity, and specificity of ultrasonography, transitional probabilities, costs, and utilities) were obtained from literature including both local and international publications, as shown in Table 1.
Table 1.
Input parameters used in health economics model.
2.3.1. Effectiveness of ultrasonography screening
The sensitivity and specificity of ultrasonography were obtained from a recent meta-analysis including 49 studies (4720 participants).[9] The overall sensitivity and specificity of ultrasound for the detection of moderate to severe fatty liver were 84.8% (95% CI: 79.5–88.9) and 93.6% (95% CI: 87.2–97.0), respectively, when compared with the gold standard of liver biopsy.
2.3.2. Effectiveness of intensive weight reduction program
The risk reduction for fibrosis progression of intensive weight reduction program was obtained from a recent prospective study of 293 biopsy-proven nonalcoholic steatohepatitis (NASH) patients in a tertiary medical care in Cuba.[12] The patients were encouraged to change their lifestyle for over 52 weeks. We based the effectiveness on this study[12] because it was the only study conducted in real-world clinical practice that explored benefits of a lifestyle intervention on fibrosis outcome. Among patients receiving lifestyle changes for 52 weeks, 88 subjects (30%) had lost 5% or more of their weight. Seventy-two subjects (25%) had achieved a resolution of steatohepatitis and 56 (19%) had regression of fibrosis. We assumed that this 52-week intensive weight reduction program was continually provided to all patients lifelong.
2.3.3. Probability data
The annual incidence of NAFLD (5.5%) was calculated based on a prospective observational study in 4401 apparently healthy Japanese at a medical health checkup program conducted in a general hospital[16] during the follow-up period of 414 ± 128 days. Regression of NAFLD was found in 16% (113 of 704 participants), who suffered from the disease at baseline and completed a second examination. NAFLD was less likely to regress in the patients with metabolic syndrome at baseline.
The transitional probabilities among all fibrosis stages (advanced, no advanced, indeterminate) were obtained from a recent meta-analysis[17] of 11 cohort studies (each with at least 1 year follow-up) of 411 biopsy-proven NAFLD patients with 2145.5 person-years of follow-up.
The transitional probability of AF progression to compensated cirrhosis and decompensated cirrhosis was obtained from 2 studies.[5,18] The transitional probability of compensated to decompensated cirrhosis was obtained from 2 studies evaluating long-term outcomes of cirrhosis in NASH compared with chronic hepatitis C,[19,20] similar to the method used in the previous cost-effectiveness study.[8]
The transitional probabilities of compensated cirrhosis to HCC and decompensated cirrhosis to HCC among NASH patients were the values used in a study by Mahady et al,[8] which were based on 3[20–22] and 4[20–23] studies, respectively. The probability of death among patients with NAFLD and cirrhosis was calculated based on the multiplication of the age-specific mortality rate (ASMR) of Thai population[24] and relative risk of mortality of NAFLD [1.29 (95% CI: 1.04–1.59, P = .020)] and cirrhosis [3.13 (95% CI: 1.08–9.12, P = .036)] compared with general population.[25] The probability of death among patients with HCC due to NAFLD was calculated based on a cohort of 287 HCC Thai patients between July 2007 and June 2010 during the follow-up period of 20.1 months.[26] These HCC patients were mostly caused by viral hepatitis B and C infections. Since NAFLD patients who have developed HCC are at risk of death due to both cancer and cardiovascular diseases, the background death rate among NAFLD needs to be added to reflect real-world mortality among this population.
2.3.4. Cost data
As the adoption of societal perspective, we included both direct medical costs and direct nonmedical costs. Indirect costs were not included as we assumed that lost or impaired ability to work or engage in leisure activities due to morbidity would be captured in the disutility of QALY.[27] All costs data were obtained from published literature in Thailand. We estimated health care utilization using microcosting technique. We assumed that patients with metabolic syndrome patients would seek outpatient visits 4 times per year and incurred costs from laboratory tests, medications for management of comorbid condition. Patients with NAFLD will be given intensive weight reduction program calculated based on cost of 4 outpatient visits per year, time for intensive counseling, and additional laboratory tests. Costs of treatment compensated cirrhosis, decompensated cirrhosis, and HCC were obtained from a large study in 5 tertiary care hospitals in Thailand.[28] The unit cost of ultrasonography was calculated based on full costing calculation at the largest hospital in Thailand in year 2014.[29] The standard unit costs for direct medical cost (laboratory testing) and direct nonmedical costs (transportation, meals, accommodation, and facilities) were obtained from a standard cost list of Thailand HTA.[30] They were converted and reported in 2014 USD (1 USD = 33 THB) and using the consumer price index (CPI).[31]
2.3.5. Utility data
All utility values were obtained from published literature. The utility of patients with metabolic syndrome was based on a large cross-sectional study evaluating HRQoL of 8941 Korean adults in the National Health Survey.[32] The mean of EUROQoL 5-dimension (EQ-5D) was.89 ± .003 in the participants with metabolic syndrome. The utility for NAFLD was.84 ± .14 based on patients with chronic liver disease.[33,34] We obtained utility data from a recently published systematic review of health-state utilities in compensated and decompensated cirrhosis states.[35] The pooled mean utility estimates in patients with compensated cirrhosis and decompensated cirrhosis using the EQ-5D were.748 and.672, respectively. In addition, we used the utility from a study of chronic hepatitis B patients for the health state of HCC, because there were no data of utility of HCC from NASH cirrhosis.[36] The utility for HCC was.38 (95% CI: 0.36–0.41).
2.4. Cost-effectiveness analysis
2.4.1. Base-case analysis
Primary outcomes of interest were the number of cases slowed down the development of cirrhosis and HCC by ultrasonography, incremental costs, QALYs gained, and ICER. For base-case analysis, we calculated the expected lifetime costs and outcomes for each program. The results are presented as ICER of ultrasonography screening with intensive weight reduction program versus no screening. Because the cohort of Thai metabolic syndrome patients had a mean age of 61 years, we used the age of ultrasonography screening at 50 years as base-case. This age was chosen to allow the screening and intensive weight reduction program to demonstrate its benefits.
2.4.2. Sensitivity analysis
One-way sensitivity analyses were performed to study the effects of altering uncertainty parameters within the 95% CI ranges including all clinical effects, costs, utilities, and discounting rate on the ICER in the model. The results of 1-way sensitivity are presented using a tornado diagram. We also varied the age for screening ranging from 30 to 80 years old. In addition, we varied the duration of the effect of intensive weight reduction program from 1 to 10 years in a sensitivity analysis. We also performed a threshold analysis to determine the lowest effectiveness value that still makes the result cost-effective. Furthermore, another threshold analysis was also conducted to determine the minimal duration of sustainable effect of intensive weight reduction program to result in cost-effective finding. A probabilistic sensitivity analysis (PSA) was also conducted to simultaneously examine the effects of all parameter uncertainties using a Monte Carlo simulation performed by Microsoft Excel 2003 (Microsoft Corp, Redmond, WA).[37] The distributions of each probability were assigned following:[38] prevalence, effectiveness of screening and intervention, probability and utility parameters, in which their values ranged between 0 and 1, were specified to beta-distributions. Costs, whose characters values above 0, were assigned to gamma distributions. Relative risk of mortality parameters were given to a log-normal distribution. A Monte Carlo simulation was run for 1000 sets of the simulation to give a range of values for total costs, outcomes, and ICERs. Results of the PSA were presented as cost-effectiveness acceptability curve. The expected net monetary benefit (NMB) was calculated for WTP threshold in Thailand to show the probability that ultrasonography screening with intensive weight reduction program is cost-effective for monetary values that a decision-maker might be willing to pay.
3. Results
3.1. Base-case analysis
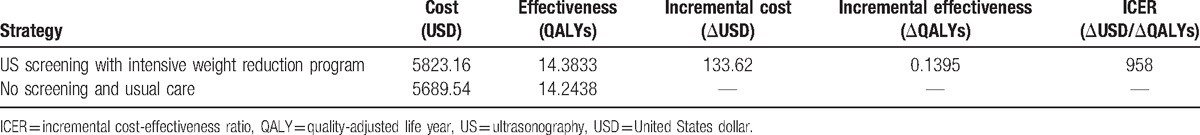
Our base-case analysis of screening age at 50 years demonstrated that ultrasonography screening with intensive weight reduction program can slow down the development of 4448 (4.45%) cirrhosis and 993 (.99%) HCC cases per 100,000 screening over lifetime. The estimated total costs for screening versus no screening were 5823.16 USD and 5689.54 USD, respectively, while the QALYs were 14.38 and 14.24 QALYs. The ICER of ultrasonography screening with intensive weight reduction program was only 958 USD per QALY gained when compared with no screening (Table 2). This is considered cost-effective based on the official willingness to pay in Thailand.[14] In addition, the results showed cost-saving in screenings at ages before 45 years and the ICER showed cost-effective in any screening age between 45 and 64 years.
Table 2.
Results of base case analysis.
3.2. Sensitivity analysis
3.2.1. One-way sensitivity analyses
Figure 2 illustrates that the most influential variables in our model were the transitional probability of NAF progression to AF, the transitional probability of AF progression to compensated cirrhosis, and effectiveness of intensive weight reduction program in fibrosis regression. Nevertheless, all parameters had no impact on cost-effectiveness interpretation for screening age at 50 years old. Our sensitivity analysis revealed that the intervention remained cost-effective as long as the risk reduction of intensive weight reduction program was larger than 11.5%. When the duration of the effect of the intervention was varied, it was still cost-effective when the effect lasted at least 7 years.
Figure 2.
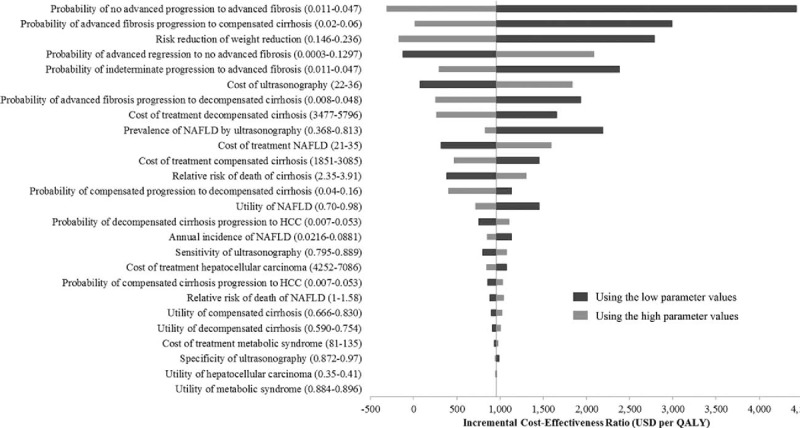
Tornado diagram of base-case analysis. This model illustrated the result of 1-way sensitivity analyses that were performed to study the effects of altering uncertainty parameters within the 95% CI ranges including all clinical effects, costs, utilities, and discounting rate on the ICER in the model. ICER = incremental cost-effectiveness ratio.
3.2.2. Multivariate probabilistic sensitivity analysis (PSA)
Results of the PSA based on 1000 Monte Carlo simulations are presented in a cost-effectiveness scatter plot below (Fig. 3A). Despite variation in base-case parameter inputs, around 45.3% of the simulated ICERs were in the lower-right quadrant, indicating that ultrasonography screening with intensive weight reduction program is less costly and more effective than no screening (dominant). Another 37.5% of ratios which lie in the upper-right hand quadrant indicate that the screening program is more effective but also more costly than no screening. Results of the PSA were also presented as a cost-effectiveness acceptability curve. The expected NMB was calculated for WTP of 4848 USD threshold in Thailand to show the probability that ultrasonography is cost-effective for monetary values that a decision-maker might be willing to pay. As shown in Fig. 3B, ultrasonography screening with intensive weight reduction program was found cost-effective in 67% of the simulations. When the starting age of screening was at 40 or 50 years, the findings are very robust and the probability of being cost-effective remains high (70% and 67%, respectively). However, the probability of being cost-effective was lower (55%) in the starting age of screening of 60 years.
Figure 3.
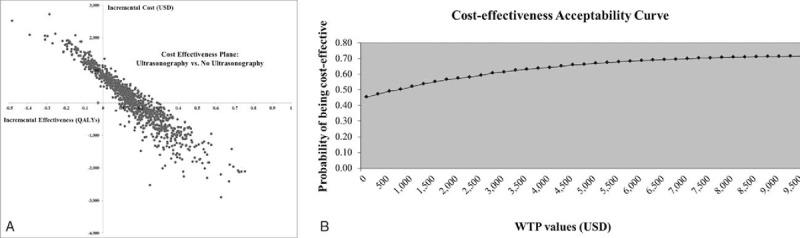
Multivariate probabilistic sensitivity analysis. The analysis was based on 1000 Monte Carlo simulations (A) cost-effectiveness plane, (B) cost-effectiveness acceptability curve.
4. Discussion
Our findings demonstrated that ultrasonography screening accompanied with intensive weight reduction among subjects with metabolic syndrome could slow down the progression to cirrhosis and HCC. It was also considered a cost-effective intervention. This impact was greater in the starting ages of screening less than 65 years. Clinicians and policy makers in Thailand may consider this program as part of national benefit package given the clear clinical and economic benefits demonstrated in this study.
Why ultrasonography screening with intensive weight reduction program is a cost-effective intervention? First, the target population was selective. We screened only high-risk population, metabolic syndrome, because the NAFLD prevalence was higher than that in general population. Second, the intervention effectively slows down costly complications. Intensive weight reduction program reduces the likelihood of developing cirrhosis and HCC which require high-cost management. The saving from slowing down such complications is further enhanced by the low cost of ultrasonography.
One of the key drivers of our cost-effective findings is the effect of intensive weight reduction program on fibrosis regression. We based the risk reduction for fibrosis progression of intensive weight reduction program on a real-world clinical practice.[12] Despite a large number of NAFLD patients not successfully achieving the goal of weight reduction, only one-third can lose weight more than 5%. Our study has taken into account the potential noncompliance of patients to this weight reduction program in the effectiveness parameter. Nevertheless, the question remains whether such an effect from the literature[12] will be generalizable to other countries including Thailand. Considering this issue, our findings need to be interpreted with caution.
To our knowledge, this study is the first study that has determined the cost-effectiveness of ultrasonography screening for NAFLD with intensive weight reduction program in patients with metabolic syndrome. We believe that our findings are highly valid and contextually relevant due to 3 main reasons. First, the hepatologist and radiologist specialists were involved throughout the process of conducting our cost-effectiveness analysis. Second, we used as much local data as possible in our analysis. We directly collected the prevalence of NAFLD in Thai metabolic syndrome patients’ data from Siriraj Hospital, Mahidol University. This had made our results more reliable in Thai context. We also adjusted the mortality rates of these patients by incorporating Thai ASMR to reflect Thai population.[39] Despite no available data of survival for NASH cirrhosis-related HCC patients, we used the combination of the ASMR of NAFLD patients with Thailand data regarding prognosis of HCC from any causes.[26] All cost data were retrieved from reliable local sources, i.e., hospital databases, National data from Ministry of Public Health (MOPH), Drugs and Medical Supplies MOPH, and the reference prices published by Thai standard cost lists for health technology assessment.[30] Moreover, we had used the cost data of treatment of cirrhotic and HCC health state from previous studies in Thailand. Third, the data of natural history of NAFLD and utilities used in the model were collected from the most recently published systematic review, meta-analysis, or other large randomized controlled trials (RCT). Moreover, we comprehensively searched literature to identify relevant probabilities, costs, and utilities, such that the model's estimates have incorporated the majority of data currently available for NAFLD.
A number of limitations in our study deserve discussion. First, ultrasonography had some limitations. Within the NAFLD spectrum, fat can both occur separately and coexist with inflammation and/or fibrosis but ultrasonography could measure only the degree of fat. In this study, we additionally used NAFLD fibrosis score (NAFLD-FS) to increase the accuracy of the model. NAFLD-FS is widely used as a noninvasive tool to predict AF and prognosis in NAFLD.[40] Second, even ultrasonography is a simple test, its implementation can be challenging in community hospitals where radiologists or gastroenterologist subspecialty is scarce. In case of ultrasonography screening performed by general practitioners, the accuracy of screening might be limited. Third, there is a paucity of health-related quality of life data among patients with NAFLD and its complications. The utility data used in our study were obtained from other chronic liver diseases. Despite feeling reasonable to assume that quality of life for end-stage liver disease is similar regardless of the causes, the validity of this assumption has not been confirmed. We performed a sensitivity analysis using a wide range of utility estimates derived from meta-analysis and other literature to determine the robustness of our findings. It was found that the results were not sensitive to changes of utility values in our model. Nevertheless, there remains a need to call for preference-based quality of life studies in patients with NAFLD in global literature.
Our findings demonstrate only that screening NAFLD in metabolic syndrome patients is cost-effective. The results are not generalizable to general population. Despite cost-effective findings, budget implication is needed to be considered before the implementation of such a program due to a large number of metabolic syndrome patients in Thailand. If we can develop other noninvasive tool as a scoring system to predict for very high-risk group in the subparticular group of metabolic syndrome population, it may decrease burden of government budget and be more effective.
It is important to discuss the generalizability of our findings for international applications. It is widely known that any cost-effectiveness findings are not directly transferable to other countries because of the differences in healthcare system, decision-making criteria, and costs data.[41] Recently, it has been advised that the potential transferability should center on process rather than findings. The potentially transferable aspects may include model structure, study design approach, and parameter values, especially those related to natural history of disease. Our paper has provided the most up-to-date information on parameters used in model. These are potentially useful to other researchers interested in this field. In addition, this is the first study evaluating the cost-effectiveness of ultrasonography for screening NAFLD in the world. We used Thailand as an example to demonstrate the value of ultrasonography screening with intensive weight reduction program in low and middle-income countries. Our findings will draw attention of clinicians and policy makers to this important issue of which global burden has been rapidly rising.
In conclusion, our study clearly shows that ultrasonography screening for NAFLD patients with weight reduction can slow down costly complications and is a cost-effective intervention in metabolic syndrome patients in Thailand, especially in the start screening age before 65 years. Policy makers may consider our findings as part of evidence-informed decision making.
Acknowledgments
The authors gratefully acknowledge the support of Dr David Wu and Dr Rosarin Sruamsiri for statistical expertise and technical support for constructing economic model.
Footnotes
Abbreviations: AASLD = American Association for the Study of Liver Diseases, AF = advanced fibrosis, ASMR = age-specific mortality rate, CPI = consumer price index, EQ-5D = EUROQoL 5-dimension, HCC = hepatocellular carcinoma, HTA = Health Technology Assessment, ICER = incremental cost-effectiveness ratio, IF = indeterminate fibrosis, MOPH = Ministry of Public Health, NAF = no advanced fibrosis, NAFLD = nonalcoholic fatty liver disease, NAFLD-FS = NAFLD fibrosis score, NMB = net monetary benefit, PSA = probabilistic sensitivity analysis, QALY = quality-adjusted life year, RCT = randomized controlled trial, ROC = receiving operating characteristics, USD = United States dollar, WTP = willingness-to-pay.
Guarantor of the article: PP.
Specific author contributions: Concept and design: PP, PC, and SS. Experiments and procedures: PP, PA, AC, CW, WS, and NC. Writing of article: PP and NC. All authors have approved the final draft submitted.
The authors have no conflicts of interest to disclose.
References
- [1].Haentjens P, Massaad D, Reynaert H, et al. Identifying non-alcoholic fatty liver disease among asymptomatic overweight and obese individuals by clinical and biochemical characteristics. Acta Clin Belg 2009;64:483–93. [DOI] [PubMed] [Google Scholar]
- [2].Vernon G, Baranova A, Younossi Z. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274–85. [DOI] [PubMed] [Google Scholar]
- [3].Phisalprapa P, Ujjin A, Apisarnthanarak P, et al. Sa1050 prevalence and risk factors of non-alcoholic fatty liver disease in Asian individuals with metabolic syndrome. Gastroenterology 2014;146:S–947. [Google Scholar]
- [4].Adams LA, Lymp JF, Sauver JS, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129:113–21. [DOI] [PubMed] [Google Scholar]
- [5].Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006;44:865–73. [DOI] [PubMed] [Google Scholar]
- [6].Söderberg C, Stål P, Askling J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology 2010;51:595–602. [DOI] [PubMed] [Google Scholar]
- [7].Musso G, Gambino R, Cassader M, et al. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med 2011;43:617–49. [DOI] [PubMed] [Google Scholar]
- [8].Mahady SE, Wong G, Craig JC, et al. Pioglitazone and vitamin E for nonalcoholic steatohepatitis: a cost utility analysis. Hepatology 2012;56:2172–9. [DOI] [PubMed] [Google Scholar]
- [9].Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology 2011;54:1082–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005–23. [DOI] [PubMed] [Google Scholar]
- [11].The Sixty-seventh World Health Assembly report 2014. Health intervention and technology assessment in support of universal health coverage. Geneva: World Health Organization; 2014. [Google Scholar]
- [12].Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015;149:367–78. [DOI] [PubMed] [Google Scholar]
- [13].Teerawattananon Y. Thai health technology assessment guideline development. J Med Assoc Thai 2008;91:S11–5. [PubMed] [Google Scholar]
- [14].Teerawattananon Y, Tritasavit N, Suchonwanich N, et al. The use of economic evaluation for guiding the pharmaceutical reimbursement list in Thailand. Z Evid Fortbild Qual Gesundheitswes 2014;108:397–404. [DOI] [PubMed] [Google Scholar]
- [15].Alberti K, Eckel RH, Grundy SM, et al. Joint scientific statement. Circulation 2009;120:1640–5. [DOI] [PubMed] [Google Scholar]
- [16].Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med 2005;143:722–8. [DOI] [PubMed] [Google Scholar]
- [17].Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015;13: 643–654.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bhala N, Angulo P, van der Poorten D, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology 2011;54:1208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hui JM, Kench JG, Chitturi S, et al. Long-term outcomes of cirrhosis in nonalcoholic steatohepatitis compared with hepatitis C. Hepatology 2003;38:420–7. [DOI] [PubMed] [Google Scholar]
- [20].Sanyal AJ, Banas C, Sargeant C, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology 2006;43:682–9. [DOI] [PubMed] [Google Scholar]
- [21].Ascha MS, Hanouneh IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010;51:1972–8. [DOI] [PubMed] [Google Scholar]
- [22].Ratziu V, Bonyhay L, Di Martino V, et al. Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology 2002;35:1485–93. [DOI] [PubMed] [Google Scholar]
- [23].Yatsuji S, Hashimoto E, Tobari M, et al. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol 2009;24:248–54. [DOI] [PubMed] [Google Scholar]
- [24].Life tables by country: Thailand, World Health organization 2013. Available at: http://apps.who.int/gho/data/view.main.61640?lang=en 2013. Accessed November 25, 2016. [Google Scholar]
- [25].Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015;61:1547–54. [DOI] [PubMed] [Google Scholar]
- [26].Leerapun A, Thaikruea L, Pisespongsa P, et al. Clinical features and prognostic factors for liver cancer from a referral center in northern Thailand. J Med Assoc Thai 2013;96:531–7. [PubMed] [Google Scholar]
- [27].Riewpaiboon A. Measurement of costs. J Med Assoc Thai 2008;91:S28–37. [PubMed] [Google Scholar]
- [28].Thongsawat S, Piratvisuth T, Pramoolsinsap C, et al. Resource utilization and direct medical costs of chronic hepatitis C in Thailand: a heavy but manageable economic burden. Value Health Regional Issues 2014;3:12–8. [DOI] [PubMed] [Google Scholar]
- [29].Unit cost of upper abdominal ultrasonography, Department of Radiology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand, 2014. [Google Scholar]
- [30].Riewpaiboon A. Standard cost lists for health technology assessment. Nonthaburi: Health Intervention and Technology Assessment Program (HITAP); 2011. [Google Scholar]
- [31].Bank of Thailand. Foreign Exchange Rates. Available at: https://www.bot.or.th/english/statistics/financialmarkets/exchangerate/_layouts/Application/ExchangeRate/ExchangeRateAgo.aspx 2014. Accessed November 25, 2016. [Google Scholar]
- [32].Lee Y-J, Woo SY, Ahn JH, et al. Health-related quality of life in adults with metabolic syndrome: the Korea national health and nutrition examination survey, 2007–2008. Ann Nutr Metab 2011;61:275–80. [DOI] [PubMed] [Google Scholar]
- [33].Younossi ZM, Boparai N, McCormick M, et al. Assessment of utilities and health-related quality of life in patients with chronic liver disease. Am J Gastroenterol 2001;96:579–83. [DOI] [PubMed] [Google Scholar]
- [34].Tongsiri S, Cairns J. Estimating population-based values for EQ-5D health states in Thailand. Value Health 2011;14:1142–5. [DOI] [PubMed] [Google Scholar]
- [35].McLernon DJ, Dillon J, Donnan PT. Health-state utilities in liver disease: a systematic review. Med Decis Making 2008;28:582–92. [DOI] [PubMed] [Google Scholar]
- [36].Levy AR, Kowdley KV, Iloeje U, et al. The impact of chronic hepatitis B on quality of life: a multinational study of utilities from infected and uninfected persons. Value Health 2008;11:527–38. [DOI] [PubMed] [Google Scholar]
- [37].Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford, UK: Oxford University Press; 2006. [Google Scholar]
- [38].Limwattananon S. Handling uncertainty of the economic evaluation result: sensitivity analysis. J Med Assoc Thai 2008;91suppl 2:S59–65. [PubMed] [Google Scholar]
- [39].Health Information Group. Bureau of Health Policy and Strategy MoPHT. Public Health Statistics A.D.2009. Available at: http://203.157.19.191/input_bps.htm. Accessed November 25, 2016. [Google Scholar]
- [40].Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846–54. [DOI] [PubMed] [Google Scholar]
- [41].Drummond M, Barbieri M, Cook J, et al. , editors. Transferability of economic evaluations across jurisdictions. International Society for Pharmacoeconomic and Outcomes Research (ISPOR)—13th International Meeting; 2008. [DOI] [PubMed] [Google Scholar]


