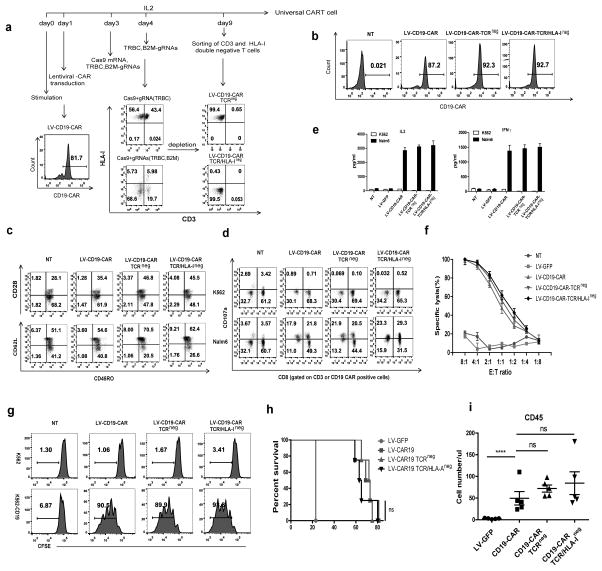
Figure 4. Generation of universal CART cells with a combination of lentiviral gene transfer and CRISPR/Cas9 electroporation.
(a) Flow chart of the generation of universal CD19-CART cells. T cells were transduced with lentiviral CD19-CAR on day 1 after stimulation, and Cas9 mRNA and gRNAs targeting the TCR β chain and B2M were electroporated in the T cells on day 3 followed by a second delivery of gRNAs on day 4. The TCR and HLA-I double-negative cell population was enriched by negative selection using microbeads on day9. (b) CD19-CAR expression of gene-modified lenti-CD19-CAR T cells. (c) Phenotype of universal CD19-CAR T cells. Function of TCR-negative and TCR/HLA-I double-negative CD19-CAR T cells tested by (d) CD107a release, (e) cytokine secretion and (f) tumor lytic capability. Representative data from 3 independent experiments are shown. Bars, SE. (g) CFSE-labeled CD19-CAR and non-transduced T cells were incubated with K562 and target K562-CD19 tumor cells at the indicated E:T ratio for 72 hours. (h) Survival curve of mice receiving gene-edited CD19-CART cells. Tumors were established in NSG mice (n=5 per group) by i.v. injection of 1×106 Nalm6 cells. Beginning on day 7, T cells (5×106) expressing lentiviral (LV) transduced CD19-CAR were infused with a single injection. T cells expressing LV GFP protein were injected as controls. ns, no difference by the log-rank Mantel-Cox test. (i) Peripheral blood from Nalm6-bearing NSG mice treated with CD19 CART cells was obtained on day 21 and quantified for the presence of CD45 T cells by a FACS Trucount assay. Results are expressed as the mean absolute count per μl of peripheral blood±SD with n=5 for all groups. ns, ****P<0.001 by Mann-Whitney test.