Abstract
The cluster-determinant 44 (CD44) receptor has a high affinity for hyaluronic acid (HA) binding and is a desirable receptor for active targeting based on its overexpression in cancer cells compared with normal body cells. The nanocarrier affinity can be increased by conjugating drug-loaded carriers with HA, allowing enhanced cancer cell uptake via the HA-CD44 receptor-mediated endocytosis pathway. In this review, we discuss recent advances in HA-based nanocarriers and micelles for cancer therapy. In vitro and in vivo experiments have repeatedly indicated HA-based nanocarriers to be a target-specific drug and gene delivery platform with great promise for future applications in clinical cancer therapy.
Keywords: hyaluronic acid, CD44, nanomicelles, anticancer drugs, targeted drug delivery, nanocarriers
Introduction
One of the greatest challenges of modern medical practice is the successful treatment of cancer, a disease forecast to have nearly 1.7 million new diagnoses in the USA in 2016 alone [1]. However, survival rates are on the rise for many cancers, and the overall 5-year cancer survival in the USA is now 69%, up from only 49% during the 1970s [1]. Yet despite the progress in this area, cancer remains a formidable disease with lower than desirable survival rates. Several fundamental challenges exist that limit the efficacy of cancer chemotherapy, namely the poor solubility of anticancer therapeutics and the limited selectivity of drug delivery within the body. The former prevents chemotherapeutics from freely circulating in the blood to reach the tumor site; the latter increases adverse effects, such as bone marrow toxicity, immune system impairment, hair loss, vomiting, and cardiotoxicity, because the drugs elicit cytotoxic effects in nontarget areas [2]. Therefore, advanced drug delivery systems are being tested to address these challenges and to find innovative means to overcome them.
One such solution is the use of nano-sized micelles as delivery vehicles for cancer therapeutics. Amphiphilic polymers self-assemble under aqueous conditions to form water-soluble micelles with a hydrophobic core. Hydrophobic drugs can then be chemically or physically incorporated into the micelle core for parenteral administration. This micelle drug delivery strategy has been applied to a variety of medical conditions, from hepatic fibrosis to knee osteoarthritis [3,4]. However, the micelle technique renders itself particularly relevant for the treatment of different types of solid tumor because of selective accumulation at the target site resulting from an enhanced permeability and retention (EPR) effect [5–7]. EPR is a phenomenon by which macromolecules, notably nanoparticles (NPs) and micelles, are delivered to, and accumulate at, the tumor site in higher concentrations than are observed in healthy tissue because of anatomical and pathophysiological abnormalities or the leaky tumor vasculature of the tumor tissues [7,8]. However, angiogenesis is not always uniform throughout a tumor, leading to disproportional drug distribution by EPR; additionally, in some cases, tumors can exist with little or no evidence of EPR [9]. Passive targeting by the EPR effect is a promising means of overcoming the specificity challenge of modern chemotherapeutic agents in many cases; furthermore, newer active targeting strategies have been developed that add to the selectivity of the EPR effect to overcome the limitations discussed above.
A key to selective toxicity in cancer therapy is to actively target cancerous tumors by exploiting the anatomical, pathophysiological, and microenvironmental differences between malignant tumors and healthy body tissue. One such difference is the expression levels of cellular uptake receptors for HA; these receptors are overexpressed in many cancer cells, especially in aggressive cancer stem cells (CSCs) or cancer stem-like cells (CSLCs) [10,11]. In particular, the CD44 receptor has a high affinity for HA binding and is a desirable receptor for the active targeting of cancers based on its overexpression in cancers compared with normal body cells [12]. Therefore, the nanocarrier affinity for cancer cells can be increased by conjugating drug-loaded micelles and/or NPs with HA, allowing enhanced uptake through the HA-CD44 receptor-mediated endocytosis pathway [11]. In this review, we discuss recent advances in HA-based micelles and nanocarriers for cancer therapy. In vitro and in vivo experiments have repeatedly indicated HA-based nanocarriers to be a target-specific drug and gene delivery platform with great promise for future applications in clinical cancer therapy.
HA and the CD44 receptors
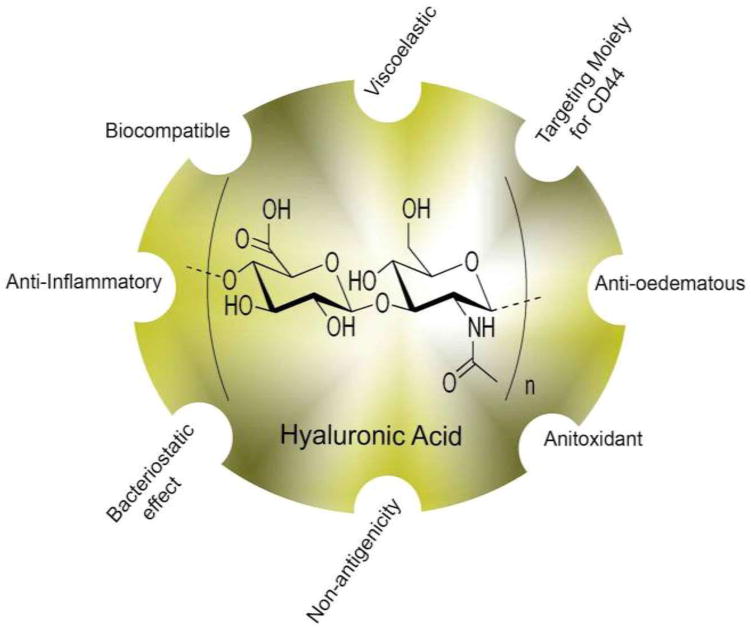
HA is a natural mucopolysaccharide that was discovered in bovine eyes by Meyer and Palmer in 1934 [13]. The structure of HA (Figure 1) has since been found to be both present and conserved in many animal species, including all vertebrates [14–16]. The molecular weight of this polymer macromolecule varies with chain length, from thousands to millions of Daltons [15,17]. HA, in combination with its receptors, is well known to be a crucial player in the extracellular matrix (ECM), contributing to angiogenesis, tissue structure, cell signaling, wound healing, and tissue hydration [15,16,18]. One principle receptor for HA is the transmembrane CD44, a glycoprotein found on the surface of many cells, and is highly overexpressed in many tumors [19]. The CD44 receptor exists in many isoforms because of variable exon splicing and post-transcriptional modifications in gene expression [20]. CD44 contains a HA-specific binding domain near the N terminus, and HA binding is an initiating step for most functions of the CD44 receptor [21,22].
Figure 1.
Hyaluronic acid (HA) structure and function. HA is a natural mucopolysaccharide that was discovered to have excellent properties for utilization in drug delivery as a targeted moiety.
HA–CD44 interactions in cancer progression
CD44 overexpression is regarded as the hallmark of the development of tumor cells, with several studies demonstrating the presence of this receptor on CSCs or tumor-initiating cells (CSCs or TICs) [23] [24,25]. HA is the main ligand for the CD44 receptor and is predominantly found in the ECM and is synthesized by HA synthase (HAS) proteins. Hyaluronidase catalyzes the degradation of HA, forming different fragments of varying molecular weights. The low-molecular-weight form of HA catabolite is known to have procancerous activity, whereas the high-molecular-weight form has anticancer activity [26]. Studies have revealed the role of HA in tumor metastasis, with HA variably expressed in different tumor milieu [27].
In addition, the interaction between HA and CD44 triggers a cascade of events that result in aggressive tumor progression and cancer metastasis. This represents an interplay between an array of signaling pathways that dictate the adhesion, metastasis, and growth of cancer cells. Such pathways resulting in cancer progression were reviewed in detail by Karbownik and Nowak [17], who explained the role of ankyrin, RhoA, Rac1, Cdc42, receptor tyrosine kinases, and MMP-9, which are activated following interactions between HA and CD44, leading to downstream signaling pathways [17]. Furthermore, Anttila et al. demonstrated the relevance of the accumulation of stromal hyaluronan in tumor progression by evaluating HA levels and expression of CD44 [28]. The authors suggested that the accumulation of HA was independent of expression of CD44 on the surface of human ovarian cancer cells [28].
Another important aspect is CD44 expression observed in both its natural and variant forms, both of which are shown to have a pivotal role in HA binding and cancer. Studies have shown the involvement of CD44 and its variants in tumor environments, underlining the role of HA binding to CD44 and its variants. A variant of CD44, CD44v6, activates the Wnt/β-catenin pathway and has a role in metastasis in colon CSCs [29]. Zhao et al. showed that CD44 is responsible for triggering cell proliferation through MAPK signaling in lung cancer cells and that a deletion in the gene encoding CD44 led to inhibition of the proliferation of Kras-mediated lung adenocarcinoma in mice [30]. In addition, HA–CD44 interactions initiate a series of events leading to binding of ankyrin to MDR-1, which in turn effluxes chemotherapeutic drugs, rendering the cancer cells chemoresistant [31]. In another study, Chen and Bourguignon suggested a pathway via which HA interacts with CD44 leading to the induction of c-Jun signaling and upregulation of Bcl-2/IAP, as a result of which chemoresistance is displayed in triple-negative breast cancer cells [32].
CD44 as a biomarker in cancer diagnosis
CD44 is a transmembrane glycoprotein with a molecular weight of 85–200 kDa, expressed on the cell surface of almost every cell in the body. Heralding the onset of cancer progression is the presence of CD44 on the surface of several CSCs [34]. It is by virtue of several tumorigenic functions portrayed by CD44 that it is considered as an early indicator for cancer cell proliferation. Hence, in a variety of cancer cells, the presence of CD44 or its isoforms can be relied upon as a biomarker for the diagnosis of cancer.
In a study by Desai et al. on prostate cancer PC3 cell lines, the expression of the CD44 variants CD44v and CD44s on the cell surface was high and was increased in PC3/OPN clones expressing high levels of full-length osteopontin [35]. The tumorigenic potential of variant splice exons of CD44 has also been studied. It was shown that CD44v6 has a pivotal role in lymph node infiltration. Furthermore, the CD44v6–10 variants have a significant role in metastasis and the development of hematological malignancies, such as lymphoma [36]. Studies establishing the relevance of CD44 as a biomarker in cancer diagnosis mainly depend on the reliability of suitable detection techniques, such as ELISA. The expression of soluble CD44 is elevated in patients with head and neck squamous cell carcinoma, and can be used as a biomarker for cancer detection [37].
The interactions between CD44 and other cell markers have been studied to determine possible mechanisms either supporting or preventing tumor growth in vivo. Together with CD44s, insulin-like growth factor 2 mRNA-binding protein 3 (IMP3) has been investigated as a biomarker for predicting the risk of hepatocellular carcinoma at initial diagnosis in cases of recurrence [38]. In another study, the correlation between expression levels of PTEN and CD44 was revealed, in which the survival of patients who were PTEN positive but CD44 negative was higher than in patients who were PTEN negative but CD44 positive [39].
In addition, Xu et al. reported the relation between higher expression levels on CD44 in basal-type breast cancer at the mRNA and protein levels, highlighting the greater dependency on CD44 as a biomarker for diagnosing basal-type breast cancer [40]. The presence of CD44v9 serves as a biomarker to detect upper tract urothelial cancer for incidences of recurrence and mortality [41], whereas CD44v6 has a crucial role in the identification of patients with distant metastasis of ovarian cancer, based on large-scale immunohistochemically analysis [42]. Not only are the variants of CD44 established as biomarkers for cancer prevalence, but they also serve as early detection tools to predict the recurrence of cancer. Studies have shown that the presence of CD44v9, a marker in primary gastric cancer tissue, has the potential to detect the recurrence of gastric cancer in patients who have been treated for multiple early gastric cancers [43]. Considering the cases described herein, it is clear that both CD44 and its variants, either alone or in interactions with another protein, have an important role in the detection of tumor cells.
HA-based micelles as a Trojan horse for cancer therapy
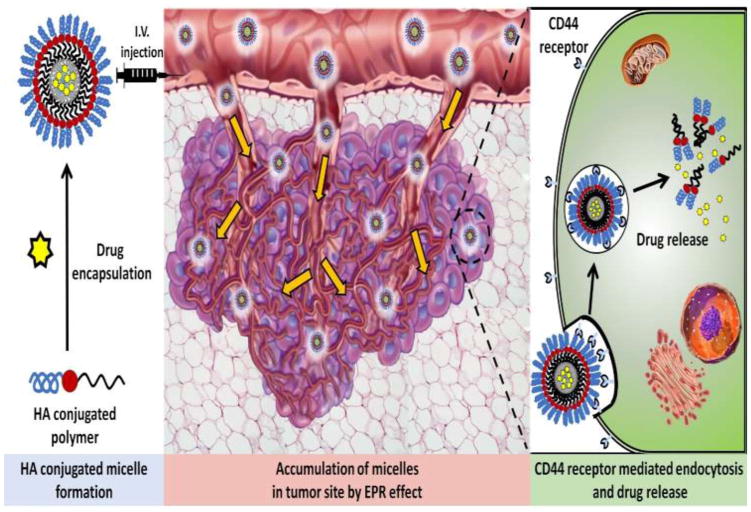
One function of the CD44 receptor is to internalize high-molecular-weight HA for degradation in the cytosol [15]. The discovery of this pathway in 1979, when HA was demonstrated for first time to bind specifically to variety of cells, was the catalyst for the development of HA-decorated micelles [44]. Attaching high-molecular-weight HA to the surface of micelles enables the latter to bind to the CD44 receptor and be internalized by the cell, as shown in Figure 2. Once encapsulated in an endosome within the cell cytosol, the micelle is disassembled and the drug is released. Lee et al. synthesized HA-based micelles from HA-PEG conjugate copolymers, linking paclitaxel (PTX) to HA by an ester bond [45]. The authors used dynamic light scattering (DLS), transmission electron microscopy (TEM), and atomic force microscopy (AFM) to confirm the self-assembly of the conjugates into micelles under aqueous conditions. Notably, Lee et al. isolated and characterized released PTX, confirming that the structure of PTX was unmodified in the encapsulation process [45].
Figure 2.
Hyaluronic acid (HA)-decorated nanomicelle-mediated active and passive targeting. The accumulation of targeted nanomicelles at the tumor site occurs via cluster-determinant 44 (CD44) receptor-mediated endocytosis. This results from the speci c binding of HA to CD44 receptors overexpressed on cancer cells. This represents active or passive targeting through the enhanced permeation and retention (EPR) effect. Abbreviation: IV, intravenous.
Three essential concerns in micelle formulation development are water solubility, particle size, and drug loading. For micelles to reach the tumor site, they must be highly soluble in water. Many anticancer agents are insoluble in free form; however, successful micelle encapsulation allows for the excellent solubility of hydrophobic drugs [7,46]. The diameter of micelles is also important because it contributes to EPR accumulation and subsequent cell permeability. Micelles with small diameters will not selectively accumulate at the tumor site by EPR, as shown in Figure 2. Large micelles (>500 nm) are unable to penetrate the cell membrane. Most micelle formulations reviewed herein have acceptable diameters in the range of 100–200 nm and were internalized via clathrin-dependent endocytosis. The third major consideration is drug loading, which is expressed as wt/wt% milligram drug per milligram overall formulation. Higher percent drug loading enables a greater concentration of chemotherapeutic agent to be released at the target site. Saadat et al. produced and optimized self-assembling targeted micelles for the in vivo transport of PTX. Their systematic approach to the optimization of encapsulation efficiency (EE%) in HA micelles revealed that EE% was inversely proportional to the drug:polymer ratio used during micelle synthesis. As Saadat et al. added more drug per milligram of HA-conjugated phospholipid polymer, they observed a lower EE% for both HA-DMPE and HA-DSPE; accordingly, they proposed that this points to a possible maximum drug-loading percentage achievable in micelle formulation [47]. Furthermore, their observed inverse correlation between the drug:polymer ratio and EE% is consistent with the results of other reports on micelle formation [48,49].
Confirmation of HA–CD44 cell entry mechanism
To evaluate the cellular mechanism used to internalize their HA-decorated micelle formulation, Qiu et al. systematically treated CD44-overexpressing cells individually with inhibitors of receptor-mediated endocytosis, inhibitors of micropinocytosis, inhibitors of caveolae-mediated endocytosis, or an HA blockade before treatment with their HPHM19 HA-decorated micelle formulation. Their results showed a strong inhibition of cellular uptake when pretreated with an excess of HA or with an inhibitor of receptor-mediated endocytosis. Uptake was decreased by 64% and 43%, respectively, as shown through fluorescent imaging. These combined data suggest that micelles utilize the interaction of HA with CD44 receptors to enter the cytosol via receptor-mediated endocytosis [50]. Similar HA blockade studies by other groups have repeatedly confirmed this finding [51–53].
Blood compatibility
Blood compatibility is crucial for micelle formulation, because formulations are administered parenterally. To this end, Pang et al. tested the potential of their HA-QT formulation for hemolytic toxicity by mixing it with red blood cells extracted from whole blood. At varying concentrations up to 100 MM, HA-QT micelle treatment resulted in less than 1% hemolysis at any concentration. The hemocompatibility of the HA-QT micelles was further confirmed by an assay of vein irritation in rabbit ears treated with the HA-QT formulation. During 3 consecutive days of administration, no irritation was observed that was any different from that occurring in the saline-treated control rabbits. Conversely, rabbits treated with free QT exhibited significant irritation, possibly because of the DMSO solvent required to dissolve the compound, which was shown to cause irritation and hemolysis in a previous study [54]. From the results of hemolysis and vein irritation studies, Pang et al. suggested HA-based micelles as a drug delivery system that successfully enhances biocompatibility compared with free-form drugs for the treatment of CD44-expressing cancers [55]. Liu et al. observed that, when compared with Taxol, PTX remained in blood circulation significantly longer when administered in HA-based micelles because of an increased half-life [56].
Cancer specificity and toxicity
HA-based micelles have been tested for efficacy against a variety of cancer cell lines with great success, including encapsulation of chemotherapeutic agents, such as PTX, doxorubicin (DOX), docetaxel, and novel drugs. The various formulations are listed in Table 1. Each of the following cancers is known to exhibit overexpression of the CD44 receptor, rendering them ideal targets for successful HA-based micelle treatment.
Table 1.
Examples of recently published research that utilizes HA as a targeting moiety
| Type of cancer | Polymer | Drug | % Loading | Micelle diameter | Target cell line | Refs |
|---|---|---|---|---|---|---|
| Breast cancer | Hexadecylamine | Docetaxel | 8–16% | 150 | MDA-MB-231 | [61] |
| Dendritic oligoglycerol | PTX | 20% | 155 | MCF-7 | [62] | |
| DOCA | PTX | 34% | 120 | MDA-MB-231 | [63] | |
| Phosphorylcholine | DOX | 10.40% | 186 | MDA-MB-231 | [74] | |
| Hydrophobic poly(L-histidine) (PH19) | DOX | 6.77% | 215 | MCF-7 | [50] | |
| Hydrophobic poly(L-histidine) (PH22) | DOX | 6.06% | 173 | MCF-7 | [50] | |
| Hydrophobic poly(L-histidine) (PH28) | DOX | 4.23% | 154 | MCF-7 | [50] | |
| Adipic dihydrazide | Quercetin | – | 172.1 | MCF-7 | [55] | |
| DOCA | PTX | 34.10% | – | MDA-MB-231 | [52] | |
| Octadecyl-grafted HA/folic acid | PTX | – | – | MCF-7 | [82] | |
| Polypropylene glycol | DOX | 2.20% | – | MCF-7 | [100] | |
| Liver cancer | Histidine/glycyrrhetinic acid | DOX | 8.64% | 162 | HepG2 | [72] |
| Poly(D,L-lactide-co-glycolide) amine | Docetaxel | 5–11% | 50–200 | HepG2 | [51] | |
| Pancreatic cancer | Copoly (styrene maleic acid) | CDF | 15.50% | 114 | MiaPaCa-2& AsPC-1 | [53] |
| Head and neck cancer | Poly(pyridyl disulfide methacrylate) | DOX | 8.70% | – | SCC7 | [76] |
| CA | PTX | 10% | – | SCC7 | [70] |
Breast cancer
Breast cancer is one of the most lethal cancers to women, second only to lung cancer. In 2015, it was the cause of 40 000 deaths in the USA [57–59]. Additionally, 12–17% of breast cancer diagnoses are triple-negative breast cancer (TNBC), an aggressive subtype that is less responsive to conventional therapeutic methods [60–62]. The CD44 receptor is overexpressed in many breast cancer subtypes and is markedly overexpressed in TNBC [60].
Zheng et al. synthesized HA-conjugated hexadecylamine micelles for the delivery of docetaxel to breast cancer cells. Their engineered HA-C16 copolymer self-assembled in an aqueous solution to form micelles of 150 nm or less, with drug loading of 8–16%. Drug uptake was then compared in vitro between CD44-positive MDA-MB-231 breast cancer cells and CD44-negative CT-26 colorectal cancer (CRC) cells after treatment with drug alone and HA-C16 micelle formulation, respectively. Both cell lines exhibited a similar content of internalized docetaxel per milligram of protein when treated with free drug; however, when treated with the same dose of docetaxel encapsulated in targeted micelles, the authors found MDA-MB-231 cells to have a docetaxel concentration greater than twice that of the CD44-negative cells and nearly four times the uptake of free docetaxel. Their results indicated that the HA conjugation of micelles successfully increased cellular uptake, utilizing the CD44 receptor pathway to gain entry to cells via receptor-mediated endocytosis [63].
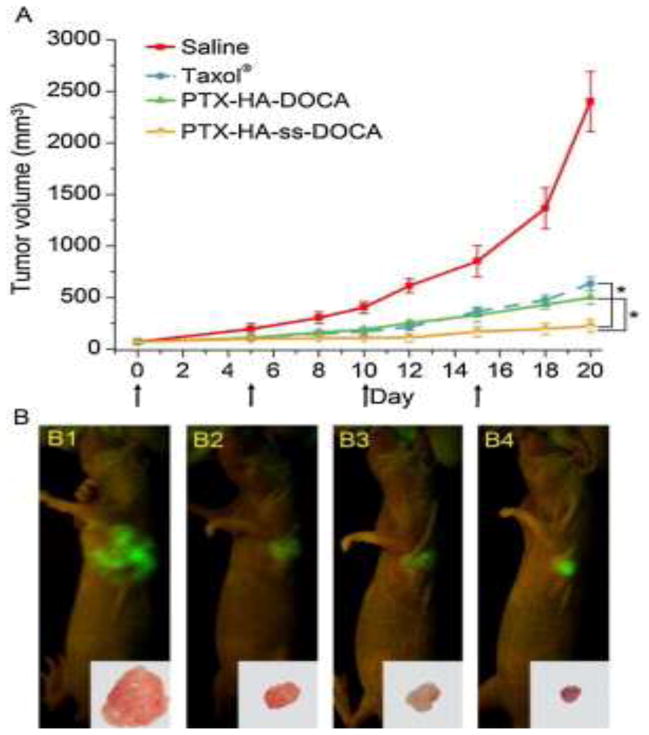
Zhong et al. demonstrated the efficacy of PTX delivery in HA-conjugated micelles for the successful treatment of MCF-7 human breast cancer in vivo by the active targeting of CD44 receptors. Targeted micelles with a measured diameter of 155 nm and 20% drug loading of PTX were self-assembled from dendritic oligoglycerol (dOG)–HA-conjugated polymers. The efficacy of their HA–dOG formulation was tested in vivo to treat mice bearing xenograft MCF-7 human breast cancer tumors overexpressing the CD44 receptor. Results showed that their engineered micelle formulation stagnated tumor progression completely, with limited adverse health effects and 100% mouse survival at 55 days. Control mice were treated with PBS during this same period, but none survived past day 45. Additionally, Zhong et al. treated mice with fluorescent DIR-loaded HA-dOG micelles and used fluorescent imaging to monitor the biodistribution after intravenous injection. Their images revealed that targeted micelles had high accumulation and retention in the tumor tissue 4–48 h after treatment; organs, such as heart, kidneys, liver, and spleen, showed lower intensity and shorter duration of micelle concentration during the same time interval. From this study, the authors concluded that HA-targeted micelles are a promising drug delivery method for the treatment of CD44-expressing tumors because of their selective accumulation at the target site [64]. Furthermore, targeted micelles with an average diameter of 120 nm were synthesized by Li et al. from a HA–deoxycholic acid (DOCA) copolymer and loaded with PTX, achieving 34% drug loading. The cytotoxicity of this formulation compared with clinically used Taxol was tested by using an MTT assay against MDA-MB-231 breast cancer cells as well as normal fibroblast (HELF) cells. PTX-loaded HA micelles showed a stronger cytotoxicity toward CD44-positive cancer cells compared with Taxol. However, when tested against HELF cells, the formulation was less toxic than Taxol at the same concentrations. The combination of these in vitro data indicates a promising formulation with both greater potencies to target tumors and increased safety for normal cells. In vivo data were concurrent with this preliminary result, as shown in Figure 3. Additionally, tests of liver and kidney function after 20 days showed greater organ damage in Taxol-treated mice than in those treated with the HA-conjugated PTX formulation. The target specificity of HA-conjugated micelles in this study suggests a favorable candidate for active tumor targeting [65].
Figure 3.
In vivo antitumor efficacy assay. (a) Tumor volumes of tumor-bearing mice as a function of time (day). The arrows indicate the time points of the drug injection. (b) Tumor-bearing mice at the end of the tests (the 20th day post-treatment) imaged by the IFLUOR™ in vivo imaging system (inset: excised tumor tissues after imaging). Paclitaxel (PTX)-hyaluronic acid (HA)-ss- deoxycholic acid (DOCA) micelles (iv) exhibited the highest tumor growth inhibition efficacy compared with saline (i), Taxol® (ii) and PTX-HA-DOCA micelles (iii). Reproduced, with permission from [65].
Colorectal cancer
CRC is currently the third most common cancer diagnosis. Women in the USA have a 4.7% probability of CRC diagnosis in their lifetime; for men, there is a 5.0% lifetime probability [66]. Several isoforms of CD44 are overexpressed in CRC tumors, and HA-based micelles have proven to be effective in vitro, rendering CRC a promising target for further HA micelle studies [67].
Lee et al. synthesized an amphiphilic HA-grafted poly [(D,L-lactic)-co-(glycolic acid)] (PLGA) polymer for the delivery of DOX to colorectal tumor carcinomas in self-assembling micelles. For DOX loading in HA-g-PLGA micelles, the authors achieved a drug loading of 7.2%. DLS and TEM were used to measure the micelle diameter, giving an average diameter of 118.2 nm for the formulation. Lee et al. used the CCK-8 assay to quantify the cell viability after treatment with either free DOX or a DOX-loaded HA-g-PLGA formulation. In HCT-116 human colon cancer cells, HA-g-PLGA NPs exhibited greater cytotoxicity than free DOX, with IC50 values of 0.67 mg DOX/ml and 3.48 mg DOX/ml, respectively. They attributed this difference in toxicity to the interaction between the HA of the micelle polymer and the overexpressed CD44 receptor in HCT-116 cells, resulting in the rapid internalization of HA-g-PLGA micelles through receptor-mediated endocytosis. Lee et al. confirmed that this pathway is utilized by the micelles through fluorescent cellular uptake studies under normal and under cold conditions (4°C). Free DOX showed similar cellular uptake in both conditions because that it passively diffuses through the membrane over time. However, DOX-loaded micelles showed a marked decrease in uptake at 4°C compared with at normal physiological temperature (37°C). Lee et al. concluded that this inhibition was a result of the energy dependence of receptor-mediated endocytosis, confirming the efficacy of the HA-targeting strategy for enhanced cellular uptake by CD44 receptors [68].
Pitarresi et al. engineered a new micelle formulation for the treatment of colon cancer based on the CD44-targeting strategy. A copolymer was formed by conjugating polylactic acid (PLA) to HA, which was then aggregated into micelles via self-assembly in an aqueous solution based on the amphiphilic nature of the molecule. Additionally, they formed HA-PLA micelles by linking to polyethylene glycol (PEG), resulting in HA-PLA-PEG micelles. Both HA-PLA and HA-PLA-PEG micelles were loaded with the anticancer drug DOX; however, further testing revealed that the HA-PLA-PEG micelles showed an increased drug loading percentage compared with the non-PEGylated formulation. Pitarresi et al. treated CD44-positive and CD44-negative cells with their developed DOX-loaded formulations to compare cytotoxicity between the two cell types. HCT-116 human colon cancer cells were used as the CD44 target because of their overexpression of the CD44 receptor, and normal human fibroblast cells were used as a comparison. After conducting a MTT cell proliferation assay, the authors concluded that both the HA-PLA and HA-PLA-PEG micelle formulations exhibited targeted cytotoxicity. The cell viability of HCT-116 colon cancer cells was significantly decreased compared with normal tissue treated with the drug-loaded micelles. Pitarresi et al. attributed this difference to an increase in micelle uptake via HA–CD44 interactions, presenting the HA-PLA formulation as a promising means of cancer treatment in vitro [69].
Liver cancer
In the USA, the incidence of, and death rates from, liver cancer have been increasing over the past 10 years [1]. Son et al. engineered CD44-targeted micelles using a PLGA polymer, the terminal carboxylic end of which was then coupled with hexamethylenediamine to achieve an amine group on which to attach HA. This HA-conjugated PLGA-amine copolymer then formed the shell of docetaxel-loaded micelles with diameters of 50–200 nm for CD44 targeting of HegG2 liver carcinoma. They achieved 50% or better drug efficiency, with drug loading of 5–11%. Results of a MTT cell viability assay after treatment with docetaxel-loaded HA micelles showed a concentration-dependent decrease in the viability of CD44-positive HepG2 liver carcinoma cells. However, when pretreated with an excess of free HA, these cells showed higher proliferation, even when treated with the same concentrations of the formulation. Son et al. proposed that the increased cell viability was because of the excess HA blocking CD44 receptors, thereby inhibiting micelle uptake via receptor-mediated endocytosis. Their results were further confirmed by tagging micelles with fluorescein isothiocyanate (FITC) and observing fluorescence after internalization. The fluorescent intensity observed in the initial treatment of HepG2 cells was more than eight times greater than when pretreated with a blockade of free HA. These observed results confirmed that HA-conjugated micelles gain cell entry utilizing the CD44 pathway, indicating a promising means of targeting cancerous liver tumors overexpressing CD44 [51].
Pancreatic cancer
Kesharwani et al. conjugated copoly(styrene maleic acid) (SMA) with HA, forming self-assembling HA-SMA micelles in aqueous solution. Targeted micelles with an average diameter of 114 nm were loaded with a novel drug, 3,4-difluorobenzylidene curcumin (CDF), to treat CD44-positive MiaPaCa-2 and AsPC-1 pancreatic cancer cell lines. Free CDF showed a high level of toxicity to pancreatic cancer cells in a MTT assay, and this potency was further increased by targeted delivery in the HA nanomicelle formulation. By comparing targeted HA-SMA-CDF micelles with nontargeted SMA-CDF micelles, the authors found that there was an increased cellular uptake and in vitro carcinoma toxicity resulting from conjugation with HA [53]. Their results indicate a potential for targeted treatment of CD44-expressing pancreatic CSCs, which are known to contribute to the resistance of a tumor to conventional cancer therapy [53,70,71]. Therefore, pancreatic cancer stem cells are an ideal target for effective therapy, and their overexpression of the CD44 receptor renders them responsive to treatment with HA-conjugated micelles.
Head and neck cancer
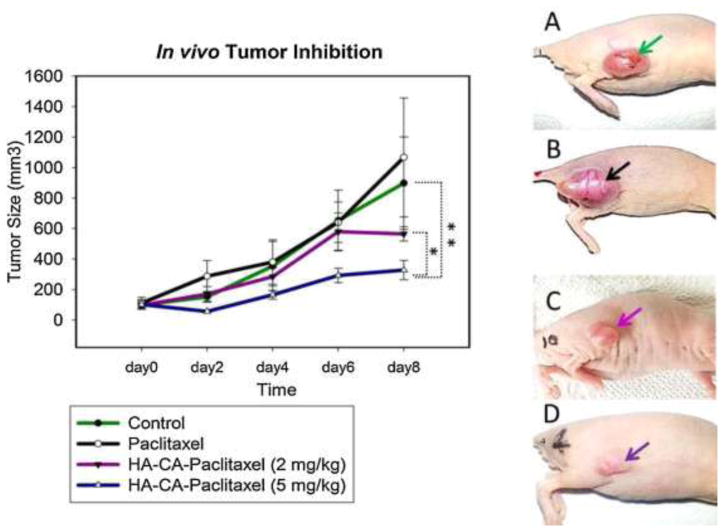
Thomas et al. synthesized and tested HA-conjugated cholanic acid (CA) micelles in delivering PTX to treat SCC7 squamous cell carcinoma [4,72]. The HA-CA formulation had a significantly greater cytotoxic effect on SCC7 cells in vitro compared with free PTX, as shown through a MTT cell proliferation assay. Thomas et al. confirmed the targeting of their formulation in vivo by tagging HA-CA micelles with a fluorescent dye and subsequently imaging the biodistribution at specific time intervals after intravenous injection into tumor-bearing mice. Their results indicated high micelle accrual at the tumor site from 0 to 2 days post injection. Importantly, most nontarget organs exhibited little or no accumulation of the targeted formulation, with only two exceptions. Accumulation was high in the liver; additionally, the authors observed some accumulation in the kidney, which is known to express CD44 [73]. Furthermore, in vivo studies of the targeted HA-CA formulation in nude mice showed significantly reduced tumor progression and less overall negative health effects compared with free PTX (Figure 4). These results indicate that HA conjugation effectively increases specific drug delivery to the target site for head and neck cancer [72].
Figure 4.
In vivo tumor inhibition study. (a) Balb/c nude mice were injected with PBS, free paclitaxel (PTX) and hyaluronic acid (HA)-cholanic acid (CA)-PTX at Day 0 (n = 3). Comparison of tumor size on Day 8 of (b) Control, (c) PTX, (d) HA-CA-PTX (2 mg/kg), and (e) HA-CA-PTX (5 mg/kg). Tumor inhibition data show mean tumor volume, of triplicate samples ± SD. *P <0.05 relative to HA-CA-PTX (2 mg/kg) and **P <0.05 relative to control group. Reproduced, with permission, from [72].
Drug release
For the systemic administration of HA micelles, drugs must be firmly encapsulated within the micelle core. Premature drug leakage leads to decreased activity at the target site as well as increased adverse effects in nontarget tissue. However, once internalized by the target cell, the percentage of drug released and the rate of release are key factors in the overall efficacy of the treatment strategy. To this end, many studies have focused on inducing drug release within the cytosol by exploiting the differences between the internal and external environments of the nanostructured micelles, or by the post-treatment application of external stimuli.
pH sensitive
Wu et al. studied the release pattern of DOX from micelles under different external pH conditions, observing that greater release was seen in more acidic environments. At a pH of 7.4, 37.9 wt % was released during the first 48 h compared with 67.9 wt % at pH 5.5. Given that 7.4 is the physiological pH of the human body, whereas 5.5 is the pH of the lysosomal environment, the authors proposed that this acid activation of drug release was favorable for drug delivery into the targeted carcinoma cells without premature drug leakage [74]. Wang et al. engineered a pH-sensitive HA micelle formulation utilizing an established click reaction concept to attach thiolated HA (HA-SH) to a side chain double bond of phosphorylcholine [75]. This copolymer was self-assembled into DOX micelles with 10% drug loading. The drug release from this formulation was studied at pH 7.4 and pH 5.0. After 48 h, the DOX release under acidic conditions (pH 5.0) was nearly double that of the release from micelles in a pH 7.4 physiological buffer [76].
Redox sensitive
Han et al. engineered a reduction-sensitive targeted micelle formulation by conjugating alkylated HA to poly(pyridyl disulfide methacrylate) via click chemistry. The crosslinked core of their micelles was to used increase the micelle stability in blood as well as to create a disulfide bond that can be reduced by glutathione (GSH) for controlled drug release at the target site. This reduction-sensitive approach is based on the known dissimilarity of GSH levels between the bloodstream (2μM) and the cell cytosol (1–10 mM) [77]. This CC-HA micelle formulation was loaded with 8.7 wt % DOX and tested against SCC7 human head and neck cancer. Drug-release studies confirmed that the DOX release time was reduced in the presence of GSH; additionally, less DOX release was observed from CC-HA micelles than their noncrosslinked HA micelle counterparts in a physiological buffer (pH 7.4). GSH levels of 10 mM induced 90% drug release from CC-HA micelles within the first 12 h of treatment. Han et al. observed strong selective accumulation of released drug at the tumor target site in vivo, because of the combination of HA targeting of CD44 receptors and GSH reduction of micelles for rapid drug release [78].
Li et al. noted that the two common shortcomings of the micelle drug delivery strategy in cancer research are lack of specificity to the target site in addition to slow release of the hydrophobic drug [79,80]. Together, these increase adverse effects while limiting the anticancer effect. To overcome these challenges, Li et al. synthesized HA-ss-XXXX (DOCA) polymers for the formation of micelles as a delivery system for the anticancer drug PTX. By conjugating with HA, they anticipated increased specificity by active targeting of CD44 receptors. By connecting the copolymer through a disulfide bond, they anticipated faster drug release induced by the reducing action of GSH in the cytosol. Li et al. achieved remarkable drug loading of PTX, with 34.1 wt% PTX at an entrapment efficiency of 93.2%. They established the ability of GSH to induce rapid PTX release from HA-ss-DOCA micelles, observing that only 6.3% of PTX was released in the first 4 h in the presence of 10μM GSH, while at 20 mM GSH, a remarkable 55.2% was released in the same time period. Li et al. confirmed the importance of the disulfide bond in this formulation by additionally synthesizing and testing HA-DOCA micelles (no disulfide connection) in the presence of the highest concentration (20 mM) GSH; in this test, only 6% of PTX was released in the first 4 h. Results of a MTT cell proliferation assay were in line with their preliminary findings, because the IC50 values after 72-h treatment with HA-ss-DOCA and HA-DOCA were 25.6 and 56.6 ng/ml, respectively. The authors used flow cytometry to determine that HA targeting increased cellular uptake in CD44-expressing MDA-MB-231 human breast cancer cells compared with MDA-MB-231 cells pretreated with an excess of HA. Thus, based on these results, Li et al. highlighted the promising potential applications of HA-ss-DOCA micelles in active targeting of CD44-positive tumors and engineered drug release in a cytosol reducing environment [52].
High-intensity focused ultrasound
Zheng et al. investigated the use of high-intensity focused ultrasound (HIFU) to control drug release from their HA-C16 micelle formulation. The impact of the focused waves of HIFU has the ability to induce drug release by disrupting the polymer bonds in the shell of polymeric micelles [81,82]. Without treatment with HIFU, drug release in vitro was observed to be a steady process during which only 37% of the drug was released over the first 120 h. When HIFU was applied, 60% of the encapsulated docetaxel was released in the first 40 h after treatment. Additionally, Zheng et al. observed an increased cellular uptake when exposed to HIFU, suggesting that HIFU increases permeability through the cell membrane. Overall, the authors found the application of HIFU to induce drug uptake and release in vitro, with possible applications in enhanced drug delivery to tumors [63].
Innovative delivery packages
In terms of cancer therapy, the complexity of cancer as a disease cannot be overstated. Cancers vary in tissue type, subtype, progression rate, and gene expression, as well as many other factors. The same initial diagnosis might require different treatment regimens in different patients. Even within the same patient, cancerous tissue might adapt over time to become unresponsive to a previously promising treatment method. This phenomenon is referred to as multidrug resistance (MDR). Therefore, the development of HA-based micelle formulations has been expanded, with several HA-micelles ingeniously designed to specifically overcome the challenges of MDR and/or P-glycoprotein (P-gp) or pump-related transporters (which otherwise would pump out the drug and decrease their effectiveness). Therefore, clinical outcomes for patients with cancer in cases of MDR will improve
Dual targeting
Liu et al. designed and synthesized HA-octadecyl (HA-C18) conjugates for the formation of self-assembling micelles for cancer treatment. Given that the expression of cellular surface receptors can adjust over time in response to drug treatment, they further conjugated with folate (FA) to form dual-targeted FA-HA-C18 micelles for the delivery of PTX, because conjugation with multiple targeting ligands has shown greater consistency in targeted delivery [83]. By targeting both the FA and CD44 receptors, the authors aimed to more effectively combat MDR even when the cellular surface morphology is altered. To this end, cytotoxicity was compared between HA-C18 single-target micelles, FA-HA-C18 dual-target micelles, and commercial Taxol solution. Their formulations were tested against MCF-7 breast cancer cells and MCF-7/Adr MDR breast cancer cells, both of which overexpress FA and HA receptors on the cell surface. Against MCF-7 human breast cancer cells, all three PTX formulations were cytotoxic at low doses, with FA-HA-C18 being the most cytotoxic at an IC50 of 0.404μg/ml. The IC50 values of HA-C18 and Taxol were 1.16 and 1.37μg/ml, respectively. However, Liu et al. observed remarkable differences in cytotoxicity against MCF-7/Adr MDR breast cancer cells. The IC50 of PTX-loaded HA-C18 micelles increased to 2.99μg/ml, whereas the dual-targeted FA-HA-C18 micelles showed 50% cytotoxicity at a PTX concentration of only 0.866μg/ml. Their results indicated a strong advantage of dual targeting to improve efficacy against MDR cells. Furthermore, the IC50 of Taxol solution against MCF-7/Adr cells was an astonishing 38.44μg/ml, suggesting that the targeted micelle drug delivery strategy drastically improves cytotoxicity to cells with MDR phenotypes [84].
In response to the low survival rates of patients with liver cancer, Wu et al. set out to synthesize a micelle drug delivery system that actively targets hepatic cancers beyond the scope of HA targeting alone. They synthesized two polymer conjugates: HA-L-histidine (Ha-His) and HA-glycyrrhetinic acid (HA-GA). From these, ultrasonic dispersion was used to form a HA-GA/HA-His mixed micelle formulation for the delivery of DOX. Wu et al. achieved 8.64% loading with an average micelle diameter of 162 nm. The active targeting strategy was twofold. HA was used to target CD44 receptors overexpressed on the cellular surface of a variety of tumor carcinomas [85,86]. Additionally, GA-conjugated polymer drug delivery systems have recently been shown to actively target the liver to halt the progression of hepatic tumors [87–89]. To evaluate their HA-GA/HA-His dual-targeting strategy, Wu et al. tested the toxicity of their DOX-loaded formulation to HepG2 liver cancer cells in vitro through the MTT colorimetric cell proliferation assay. Results showed that dual-targeted micelles had greater toxicity than free DOX, with IC50 values of 1.19 and 1.46μg/ml, respectively [74].
Liu et al. engineered a dual-targeting micelle drug delivery system by conjugation of octadecyl-grafted HA (HA-C18) with folic acid (XX) to produce FA-HA-C18 polymers for the micelle encapsulation of PTX. Cellular uptake of PTX in drug-loaded micelles was fourfold greater than with the commercial formulation Taxol in both MCF-7 human breast cancer (overexpression of HA and FA receptor) and A549 human lung cancer (overexpression of HA receptor) cells. In a comparison of the cellular uptake between single-targeted and dual-targeted micelles, Liu et al. observed a 36.7% increase in uptake of FA-HA-C18 micelles over HA-C18 micelles in MCF-7 cells. However, in A549 cells exhibiting overexpression of only the HA receptor, a significant difference in uptake was not observed. MTT assay results showed that dual-targeted micelles were more cytotoxic than both HA-C18 micelles and the Taxol to MCF-7 cell line at all concentrations tested between 0.001 and 100 MM PTX. In CD44-overexpressing A549 cells, HA-C18 and FA-HA-C18 micelle formulations were more cytotoxic than Taxol at low concentrations only (<1 MM). By combining HA micelles with additional FA targeting, Liu et al. showed increased cellular uptake and subsequent cytotoxicity for improved treatment of certain receptor-overexpressing cell lines, such as MCF-7 breast cancer [90].
Drug–gene combinations
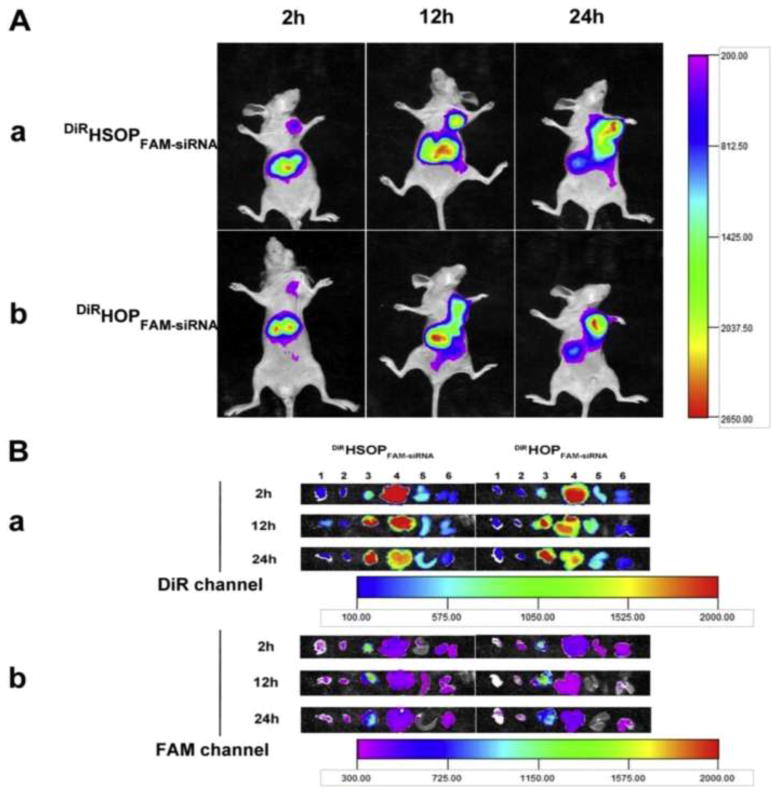
Yin et al. utilized HA tumor-targeted micelles for the codelivery of the anticancer drug PTX and small interfering (si)RNA specific to the inhibition of aurora kinase A (AURKA) to treat aggressive triple-negative breast cancer. Recently, siRNA was used with substantial success in silencing cancerous gene expression to treat various carcinomas [91]. Additionally, previous studies have revealed that the inhibition of AURKA in breast cancer cells increases their susceptibility to the cytotoxicity of the drug class taxanes, among which are docetaxel and PTX [92–94]. The MTT cell viability assay confirmed the synergistic effects of the dual-loaded HA micelle therapy against CD44-positive MDA-MB-231 breast cancer cells. In vivo fluorescent studies of tumor-bearing mice confirmed the selective tumor targeting of the target formulation. By codelivery of AURKA inhibitors and PTX in HA micelles, Yin et al. showed the significant anticancer effect of their drug–gene dual treatment formulation (Figure 5) [95].
Figure 5.
(a) In vivo imaging of tumor-bearing mice after administration of DiR/FAM-small interfering RNA (siRNA) co-loaded HSOP micelles (i) and HOP micelles (ii) at 2 h, 12 h, and 24 h under DiR channel (720 nm for excitation and 790 nm for emission). (b) Ex vivo fluorescence images of tissues including: lung (1), heart (2), tumor (3), liver (4), spleen (5), and kidneys (6) collected at 2 h, 12 h and 24 h post-injection of DiR/FAM-siRNA co-loaded HSOP micelles (DiRHSOPFAM-siRNA) and HOP micelles (DiRHOPFAM-siRNA) under DiR channel [(i) 720 nm for excitation and 790 nm for emission) and FAM channel [(ii) 470 nm for excitation and 530 nm for emission), respectively. Reproduced, with permission, from [95].
Yang et al. synthesized and characterized HA-PEI/HA-PEG NPs. These HA-based self-assembling NPs can target CD44 receptors overexpressed on MDR ovarian cancer. The authors then evaluated the cellular uptake and knockdown efficiency of HA-PEI/HA-PEG/MDR1 siRNA NPs and found that HA-PEI/HA-PEG NPs successfully targeted CD44 and delivered MDR1 siRNA into OVCAR8TR (established PTX-resistant) tumors. In addition, HA-PEI/HA-PEG NPs loaded with MDR1 siRNA efficiently downregulated the expression of MDR1 and Pgp. Thus, cell sensitivity to PTX was increased and HA-PEI/HA-PEG/MDR1 siRNA NP therapy followed by PTX treatment inhibited tumor growth in MDR ovarian cancer mouse models [96,97]. These results indicated that this CD44 targeted HAPEI/HA-PEG NP nanostructured might be a clinically relevant gene delivery system for systemic siRNA-based anticancer therapeutics for the treatment of MDR ovarian cancers.
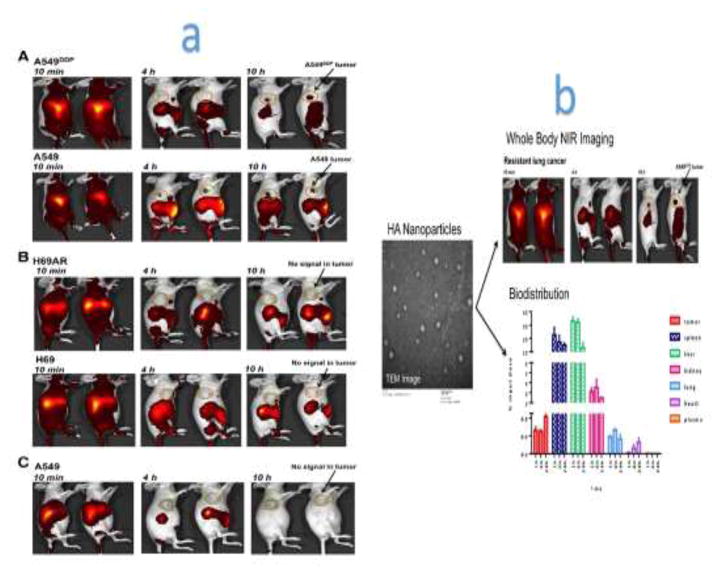
In another study, Ganesh et al. developed a near infrared (NIR) dye-loaded HA NP, which was applied to image the whole-body localization of NPs after intravenous injection into live mice bearing human lung tumors that were sensitive and resistant to cisplatin. Ganesh et al. quantified the siRNA duplexes and cisplatin dose distribution in various tissues and organs using an ultrasensitive quantitative PCR method and inductively coupled plasma-mass spectrometry (ICP-MS) (Figure 6). They found that the distribution pattern of the siRNA and cisplatin using customized engineered CD44-targeting HA NPs corresponded well with the tumor targeting capability as well as the activity and efficacy procured from combination treatments [98].
Figure 6.
(a) Whole-body optical imaging. The distribution of indocyanine green-encapsulated hyaluronic acid (HA)-poly(ethylenimine)/HA-poly(ethylene glycol) self-assembled nanoparticles (ICG/HA-PEI/PEG NP) in A549/A549DDP non-small cell lung cancer- (i) and H69/H69AR small cell lung cancer- (ii) bearing mice. Mice bearing A549 andA549DDP and H69/H69AR tumors were injected with ICG/HA-PEI/PEG NPs and imaged at different time points using the IVIS live imaging system. To see the half-life of ICG alone in circulation, the free dye ICG was injected into A549 tumor-bearing mice and imaged at different time points (iii). (b) Biodistribution of NPs throughout different organs in the mice tissues. Reproduced, with permission, from [98].
SPIONs
Smejkalova et al. engineered a novel approach to micelle drug delivery by combining HA micelles with superparamagnetic iron oxide NPs (SPIONs). They synthesized a sodium oleyl hyaluronate (HAC18:1) amphiphilic polymer to encapsulate hydrophobic oleic acid-coated SPIONs into self-assembling micelles. Interestingly, HAC18:1 micelles loaded with 1–2% SPION were cytotoxic to the cancer cell line HT-29 selectively over normal human cells in a MTT assay. Notably, SPION treatment was more selective to cancer cells than was the HAC18:1 PTX-loaded micelle. This apparent selectivity of SPIONs was further confirmed by observing the selectivity of the effect of the treatment on HT-29 cancer cells and normal cells when mixed together. Smejkalova et al. attributed this selectivity in part to observations of different cellular release mechanisms in the two cell lines. SPIONs were imaged in clusters within the cytoplasm of normal cells; however, they did not aggregate in cancer cells [99].
Active polymers
One known aspect of MDR in a variety of cancers is the action of P-gp in pumping an internalized drug from within the cytoplasm back to the extracellular space. Thus, Qiu et al. synthesized self-assembling micelles from HA-g-poly(I-histidine) copolymers mixed with d-α-tocopheryl polyethylene glycol 2000 (TPGS2k). Incorporation of TPGS (2%) into the targeted micelles was used. to inhibit the action of P-gp, thus allowing for both the delivery and retention of active drug. Their mixed HPHM/TPGS2k micelles were loaded with DOX at 9.93 wt % DOX. Furthermore, DOX-loaded HPHM/TPGS2k micelles were found to be more cytotoxic than were HPHM (non-TPGS2k) micelles to MDR MCF-7/ADR cancer cells in vitro. Qiu et al. ascribed this observation to the successful inhibition of P-gp drug efflux by TPGS2k [100].
In another study, Jung et al. synthesized a copolymer polypropylene glycol-HA (PPG-g-HA) conjugate, forming mixed micelles from the combination of a PPG-g-HA polymer and pluronic L61. Given its low hydrophilic-lipophilic balance, pluronic acid might act in the cytosol and cell membrane to reduce ATP levels and combat MDR, making it a desirable vehicle for drug delivery [101]. DOX-loaded PPG-g-HA and PPG-g-HA/L61 micelles were formed and analyzed with 0%, 3%, 15%, or 40% pluronic L61 to determine the optimal percentage of pluronic L61 to be incorporated into the micelle structure. The authors found that 3% pluronic L61 increased micelle stability and allowed for greater drug loading compared with other formulations. PPG-g-HA and PPG-g-HA/L61-3% formulations were selected for further cytotoxic analysis, with a DOX loading of 2.2% and 3.1%, respectively. Treatment of L929 normal human fibroblast cells with DOX-loaded micelles showed no significant decrease in viability at low concentrations, whereas treatment with free DOX at the same concentrations was highly cytotoxic. Furthermore, Jung et al. found that the treatment of a MCF-7 MDR breast cancer cell line with drug-loaded formulations showed that PPG-g-HA/L61 exhibited greater cytotoxicity than did PPG-g-HA micelles, suggesting that pluronic L61 contributed to the cytotoxicity in MCF-7 cells. The greatest toxicity to MCF-7 was affected by treatment with free DOX; however, free DOX is neither specific to the target site, as shown by its high toxicity to L929 normal cells, nor soluble for parenteral administration. Therefore, PPG-g-HA/L61 micelles were the most selective cytotoxic delivery system tested by Jung et al. for the selective treatment of cancer cells. Furthermore, by mixing the functional pluronic L61 polymer with CD44-targeted PPG-g-HA copolymers, Jung et al. were able to create a novel formulation that utilizes both the targeting strategy of HA and the ability of pluronic L61 to combat MDR cancers via ATP depletion [102].
Recent patents with HA as the targeting moiety
US Patent no. 7,897,584 B2, issued to De Luca et al. [103], describes the water-soluble taxanes covalently bonded to HA or HA derivatives, and in particular to PTX and docetaxel, which are useful for the preparation of pharmaceutical compositions to be used in the field of oncology in the treatment of autoimmune disorders and restenosis. As stated in the patent, the authors also discussed the process for preparing taxanes covalently bonded to HA or HA derivatives by direct synthesis between molecules of HA and taxane or by indirect synthesis by the introduction of a spacer between the HA or HA derivative and the taxane. This invention has many advantages, including the provision of instantaneous solubility in the blood stream. In addition, there is no need to mix taxenes with cremophore®EI, which causes adverse effects; and the conjugate will take advantage of esterase enzymes that are commonly present in plasma for drug release.
As per patent US 8,895,069 B2 [104], Hahn et al. provided a drug delivery composition comprising drug-loaded, HA-peptide conjugate micelles applicable to hydrophobic or water-insoluble drugs. Moreover, this micelle has a therapeutic effect because of the peptide it contains, which can act in combination with the drug. Thus, the drug delivery system and its production method can be utilized to produce a sustained-release formulation with an extended duration of the medicinal effect.
According to patent US 2005/0123505 A1 [105], a biodegradable HA derivative when dissolved in a hydrophilic medium can form micelles and can be used to entrap a pharmaceutically active or bioactive molecule. This patent highlights the process of making a novel biodegradable HA derivative by substituting a short chain group on the hydroxyl position via a urethane linkage.
In addition, Hunter et al. reported in US 2006/0040894 A1 patent, compositions, devices, and methods for prolonging the activity of HA-based platforms, which can be utilized in the drug delivery, cancer therapy, and the treatment of interstitial cystitis [106]. They provided examples of HA-containing materials for use in drug delivery, which can be combined with an HA-including Hyaluronic-Induced Targeting (HIT) gel, such as the NASHA gel (Q-Med) from SkyePharma. Also, topical formulations, such as SOLARESE and SOLARASE, from Meditech (Australia), are gels used in the treatment of skin cancer.
Patent US 2010/0316682 A1 [107] disclosed a biodegradable HA derivative including at least one modified HA repeating unit represented by the formula (HA)- [O(C:O) NH-M]p, in which HA is a unit including N-acetyl-D-glucosamine and D-glucuronic acid, M is a modifying moiety containing a C2-16 hydrocarbyl group, and p is an integer of 1–4. Furthermore, Yang et al., in US patent no. 2014/0199349 A1, disclosed a pH-sensitive HA derivative, comprising at least one repeat unit in which the HA derivative is biodegradable and pH sensitive, and linkages are broken under acidic conditions [108]. The disclosure also provides a micelle formed in the hydrophilic medium. Furthermore, it provides a drug delivery system that comprises a carrier and a bioactive ingredient encapsulated within the carrier. In addition, the disclosure provides a flavor enhancer for encapsulating a bioactive to mask the flavor thereof, in which the flavor enhancer comprises the new HA derivative. In addition, Amiji and Iyer in US20110244048 A1 patent developed libraries of NPs comprising therapeutic agents and/or imaging agents, as well as disclosing methods of making, customizing, and using HA as one example of targeting a ligand to CD44 [109].
HA in preclinical and clinical cancer trials: challenges and promises
HA is a promising molecule for utilization as a vehicle, which can circumvent problems faced by conventional cancer chemotherapy. It has also been utilized as a targeting moiety, because it can target and bind to the CD44 receptor overexpressed in several cancers. In terms of preclinical and/or clinical trials, the most recent drug delivery technologies are polymer drug conjugates and injectable formulations. As shown in the literature, several products have reached Phase 2 or Phase 3 clinical trials. One of the hurdles for HA conjugates for their utility in cancer therapeutics is because of their classification as new chemical entities (NCEs); therefore, physicochemical characterizations, such as the drug release patterns, drug loading, particle size, and morphology, must be optimized. Most of the clinical data have shown a remarkable reduction of adverse effects, but their efficacy still needs to be improved significantly. One option might be to enhance the activity of the HA conjugates, particularly to overcome tumor MDR, by coloading or linking more toxic and/or potent anticancer agents. To this end, suitable linkers to fine tune drug release will be mandatory to enhance the therapeutic outcomes further. Multiple anticancer drugs (e.g., irinotecan, DOX, 5FU, and MTX) have been investigated in clinical trials by utilizing HA as a targeting moiety [19]. A subset of these preclinical data revealed modest anticancer efficacy and an improved safety profile. Also, Phase 1 clinical trials have been conducted on 12 patients and the results showed that HA-irinotecan was safe and well tolerated, and that the anticancer activity of irinotecan was not compromised [19]. Another Phase 2 trial, involving 41 patients, emphasized the advantages of the HA nanoformulation in terms of progression-free survival and safety [20]. In 2014, another Phase 2 trial of 5-FU and HA-irinotecan plus cetuximab, was initiated (NCT02216487) by Alchemia Oncology. This trial aims to investigate the use of HA-Irinotecan in a single-arm trial of FOLF(HA) plus cetuximab in irinotecan-naïve second-line patients with KRAS wildtype metastatic CRC. Also, these studies aim to confirm the safety and efficacy of FOLF(HA) plus cetuximab as second-line therapy in irinotecan-naïve patients with metastatic (m)CRC. Table 2 provides examples of ongoing trials in which HA is utilized as an excipient and/or targeting ligand in cancer drug delivery.
Table 2.
Examples of current ongoing clinical trials utilizing HA as excipient/targeting ligand in cancer drug delivery
| Year (NCT) | Clinical trial | Intervention | Condition |
|---|---|---|---|
| 2014 (NCT02216487) | Trial of FOLF(HA)Iri with cetuximab in mCRC | HA-Irinotecan | mCRC |
| 2016 (NCT02753595) | Study of eribulin mesylate in combination with PEGylated recombinant human hyaluronidase (PEGPH20) versus eribulin mesylate alone in subjects with (HER2)-negative, high-hyaluronan (HA) metastatic breast cancer (MBC) | Drug: eribulin mesylate; biologic: PEGylated recombinant human hyaluronidase (PEGPH20) | Metastatic breast cancer |
| 2015 (NCT02346370) | A Phase 1b study of PEGylated recombinant human hyaluronidase (PEGPH20) combined with docetaxel in subjects with recurrent previously treated locally advanced or metastatic non-small lung cancer | Drug: PEGPH20; drug: docetaxel | Non-small cell lung cancer |
| 2016 (NCT02715804) | A study of PEGylated recombinant human hyaluronidase in combination with Nab-PTX plus gemcitabine compared with placebo plus Nab- PTX and gemcitabine in participants with hyaluronan-high stage IV previously untreated pancreatic ductal adenocarcinoma | Biological: PEGylated recombinant human hyaluronidase (PEGPH20); drug: placebo; drug: Nab-PTX; drug: gemcitabine | Pancreatic ductal adenocarcinoma |
| 2016 (NCT02910882) | PEGPH20 plus gemcitabine with radiotherapy in patients with localized, unresectable pancreatic cancer | Drug: PEGylated recombinant human hyaluronidase (PEGPH20); drug: Gemcitabine; radiation: radiation | Pancreatic adenocarcinoma (nonresectable) |
Concluding remarks and prospects
The incorporation of HA onto the exterior surface of micelles and NPs has repeatedly shown improvement in the targeting of therapeutic agents to cancer cells in vitro and in vivo. In combination with the passive targeting or the EPR effect, this active targeting strategy is a promising platform for delivering chemotherapeutic drugs to CD44-overexpressing cancers. In particular, this technique enables the targeting of CSCs or CSLCs and aggressive cancer cells that contribute to cancer proliferation and metastasis. HA-based nanomicelles and nanocarriers are biologically safe for drug delivery and show great potential for drug loading, blood compatibility, and systemic tumor targeting. Additionally, recent advances in HA-based nanoformulation developments have shown improvement in combating multidrug resistance. Together, these data make a strong case for the continuation of research into the use of HA for future clinical cancer therapy.
Highlights.
CD44 receptor is overexpressed in highly invasive stem-like cancer cells (SLCS)
CD44 receptors have high affinity towards hyaluronic acid (HA) binding.
Recent advances using HA based nanocarriers for cancer therapy are discussed.
HA decorated nanocarries shown great promise for future applications in cancer therapy.
Acknowledgments
J.M.W. would like to acknowledge the Department of Pharmaceutical Sciences, Wayne State University (WSU), for a summer undergraduate research fellowship (SURF) in the Iyer lab. A.K.I. acknowledges WSU start-up funding for research support. The authors wish to acknowledge Sara Tipton for editorial assistance.
Footnotes
Teaser: Hyaluronic acid (HA) has potential as a unique targeting ligand for treating multiple types of tumor overexperssing cluster-determinant 44 (CD44) receptors, including stem-like cancer cells (SLCC). Here, we discuss the innovative approaches of HA-decorated nanocarriers developed by researchers for delivering drugs and genes to cancer cells.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, et al. Cancer statistics. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Fox ME, et al. Soluble polymer carriers for the treatment of cancer: the importance of molecular architecture. ACC Chem Res. 2009;42:1141–1151. doi: 10.1021/ar900035f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayol L, et al. Amphiphilic hyaluronic acid derivatives toward the design of micelles for the sustained delivery of hydrophobic drugs. Carbohydr Polym. 2014;102:110–116. doi: 10.1016/j.carbpol.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Thomas RG, et al. Effectiveness of losartan-loaded hyaluronic acid (HA) micelles for the reduction of advanced hepatic fibrosis in C3H/HeN mice model. PLoS ONE. 2015;10:e0145512. doi: 10.1371/journal.pone.0145512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsumura Y, Maeda H. A New concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent Smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 6.Iyer AK, et al. Polymeric micelles of zinc protoporphyrin for tumor targeted delivery based on EPR effect and singlet oxygen generation. J Drug Target. 2007;15:496–506. doi: 10.1080/10611860701498252. [DOI] [PubMed] [Google Scholar]
- 7.Iyer AK, et al. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Greish K. Enhanced permeability and retention effect for selective targeting of anticancer nanomedicine: are we there yet? Drug Discov Today Technol. 2012;9:e71–e174. doi: 10.1016/j.ddtec.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Peer D, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 10.Takaishi S, et al. Identification of Gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006–1020. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaracz S, et al. Recent advances in tumor-targeting anticancer drug conjugates. Bioorg Med Chem. 2005;13:5043–5054. doi: 10.1016/j.bmc.2005.04.084. [DOI] [PubMed] [Google Scholar]
- 12.Qhattal HSS, Liu X. Characterization of CD44-mediated cancer cell uptake and intracellular distribution of hyaluronan-grafted liposomes. Mol Pharm. 2011;8:1233–1246. doi: 10.1021/mp2000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer K, Palmer JW. The polysaccharide of the vitreous humor. J Biol Chem. 1934;107:629–634. [Google Scholar]
- 14.Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999;7:79–89. doi: 10.1046/j.1524-475x.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 15.Mattheolabakis G, et al. Hyaluronic acid targeting of CD44 for cancer therapy: from receptor biology to nanomedicine. J Drug Target. 2015;23:605–618. doi: 10.3109/1061186X.2015.1052072. [DOI] [PubMed] [Google Scholar]
- 16.Necas J, et al. Hyaluronic acid (hyaluronan): a review. Vet Med. 2008;53:397–411. [Google Scholar]
- 17.Karbownik MS, Nowak JZ. Hyaluronan: towards novel anti-cancer therapeutics. Pharmacol Rep 2013. 2013;65:1056–1074. doi: 10.1016/s1734-1140(13)71465-8. [DOI] [PubMed] [Google Scholar]
- 18.Oh EJ, et al. Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. J Control Release. 2010;141:2–12. doi: 10.1016/j.jconrel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Choi KY, et al. Hyaluronic acid-based nanocarriers for intracellular targeting: interfacial interactions with proteins in cancer. Colloids Surf B Biointerfaces. 2012;99:82–94. doi: 10.1016/j.colsurfb.2011.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackay CR, et al. Expression and modulation of CD44 variant isoforms in humans. J Cell Biol. 1994;124:71–82. doi: 10.1083/jcb.124.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banerji S, et al. Structures of the Cd44–hyaluronan complex provide insight into a fundamental carbohydrate-protein interaction. Nat Struct Mol Biol. 2007;14:234–239. doi: 10.1038/nsmb1201. [DOI] [PubMed] [Google Scholar]
- 22.Naor D, et al. CD44 in cancer. Crit Rev Clin Lab Sci. 2002;39:527–579. doi: 10.1080/10408360290795574. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh SC, et al. CD44: a validated target for improved delivery of cancer therapeutics. Expert Opin Ther Targets. 2012;16:635–650. doi: 10.1517/14728222.2012.687374. [DOI] [PubMed] [Google Scholar]
- 24.Orian-Rousseau V. CD44, a therapeutic target for metastasising tumours. Eur J Cancer. 2010;46:1271–1277. doi: 10.1016/j.ejca.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 25.Sugahara KN, et al. Chondroitin sulfate E fragments enhance CD44 cleavage and CD44-dependent motility in tumor cells. Cancer Res. 2008;68:7191–7199. doi: 10.1158/0008-5472.CAN-07-6198. [DOI] [PubMed] [Google Scholar]
- 26.Naor D. Editorial: interaction between hyaluronic acid and its receptors (CD44, RHAMM) regulates the activity of inflammation and cancer. Front Immunol. 2016;7:39. doi: 10.3389/fimmu.2016.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tammi RH, et al. Hyaluronan in human tumors: pathobiological and prognostic messages from cell-associated and stromal hyaluronan. Semin Cancer Biol. 2008;18:288–295. doi: 10.1016/j.semcancer.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Anttila MA, et al. High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer high levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res. 2000;60:150–155. [PubMed] [Google Scholar]
- 29.Todaro M, et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell. 2014;14:342–356. doi: 10.1016/j.stem.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Zhao P, et al. CD44 promotes Kras-dependent lung adenocarcinoma. Oncogene. 2013;32:5186–5190. doi: 10.1038/onc.2012.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bourguignon LYW, et al. Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J Biol Chem. 2008;283:17635–17651. doi: 10.1074/jbc.M800109200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L, Bourguignon LYW. Hyaluronan-CD44 interaction promotes c-Jun signaling and miRNA21 expression leading to Bcl-2 expression and chemoresistance in breast cancer cells. Mol Cancer. 2014;13:52. doi: 10.1186/1476-4598-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basakran NS. CD44 as a potential diagnostic tumor marker. Saudi Med J. 2015;36:273–279. doi: 10.15537/smj.2015.3.9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desai B, et al. Mechanisms of osteopontin and CD44 as metastatic principles in prostate cancer cells. Mol Cancer. 2007;6:18. doi: 10.1186/1476-4598-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akisik E, et al. CD44 variant exons in leukemia and lymphoma. Pathol Oncol Res. 2002;8:36–40. doi: 10.1007/BF03033699. [DOI] [PubMed] [Google Scholar]
- 37.Franzmann EJ, et al. Soluble CD44 is a potential marker for the early detection of head and neck cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:1348–1355. doi: 10.1158/1055-9965.EPI-06-0011. [DOI] [PubMed] [Google Scholar]
- 38.Hu S, et al. IMP3 combined with CD44s, a novel predictor for prognosis of patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2014;140:883–893. doi: 10.1007/s00432-014-1639-x. [DOI] [PubMed] [Google Scholar]
- 39.Huang X, et al. Prognostic value of the expression of phosphatase and tensin homolog and CD44 in elderly patients with refractory acute myeloid leukemia. Oncol Lett. 2015;10:103–110. doi: 10.3892/ol.2015.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu H, et al. Enrichment of CD44 in basal-type breast cancer correlates with EMT, cancer stem cell gene profile, and prognosis. Oncol Targets Ther. 2016;9:431–444. doi: 10.2147/OTT.S97192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagiwara M, et al. Variant isoforms of CD44 expression in upper tract urothelial cancer as a predictive marker for recurrence and mortality. Urol Oncol Semin Orig Investig. 2016;34:337.e19–337. doi: 10.1016/j.urolonc.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 42.Motohara T, et al. CD44 variant 6 as a predictive biomarker for distant metastasis in patients with epithelial ovarian cancer. Obstet Gynecol. 2016;127:1003–1011. doi: 10.1097/AOG.0000000000001420. [DOI] [PubMed] [Google Scholar]
- 43.Hirata K, et al. CD44 variant 9 expression in primary early gastric cancer as a predictive marker for recurrence. Br J Cancer. 2013;109:379–386. doi: 10.1038/bjc.2013.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alho AM, Underhill CB. The hyaluronate receptor is preferentially expressed on proliferating epithelial cells. J Cell Biol. 1989;108:1557–1565. doi: 10.1083/jcb.108.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee H, et al. Hyaluronic acid-paclitaxel conjugate micelles: synthesis, characterization, and antitumor activity. Bioconjug Chem. 2008;19:1319–1325. doi: 10.1021/bc8000485. [DOI] [PubMed] [Google Scholar]
- 46.Kesharwani P, et al. Parenterally administrable nano-micelles of 3, 4-difluorobenzylidene curcumin for treating pancreatic cancers. Colloids Surf B Biointerfaces. 2015;132:138–145. doi: 10.1016/j.colsurfb.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Saadat E, et al. Synthesis and optimization of a novel polymeric micelle based on hyaluronic acid and phospholipids for delivery of paclitaxel, in vitro and in-vivo evaluation. Int J Pharm. 2014;475:163–173. doi: 10.1016/j.ijpharm.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 48.Dabholkar RD, et al. Polyethylene glycol–phosphatidylethanolamine conjugate (PEG–PE)-based mixed micelles: some properties, loading with paclitaxel, and modulation of P-glycoprotein-mediated efflux. Int J Pharm. 2006;315:148–157. doi: 10.1016/j.ijpharm.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 49.Tao Y, et al. Core cross-linked hyaluronan-styrylpyridinium micelles as a novel carrier for paclitaxel. Carbohydr Polym. 2012;88:118–124. [Google Scholar]
- 50.Qiu L, et al. Self-assembled pH-responsive hyaluronic acid-g-poly((L)-histidine) copolymer micelles for targeted intracellular delivery of doxorubicin. Acta Biomater. 2014;10:2024–2035. doi: 10.1016/j.actbio.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 51.Son GM, et al. Self-assembled polymeric micelles based on hyaluronic acid-g-poly(D,L-lactide-co-glycolide) copolymer for tumor targeting. Int J Mol Sci. 2014;15:16057–16068. doi: 10.3390/ijms150916057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li J, et al. Redox-sensitive micelles self-assembled from amphiphilic hyaluronic acid-deoxycholic acid conjugates for targeted intracellular delivery of paclitaxel. Biomaterials. 2012;33:2310–2320. doi: 10.1016/j.biomaterials.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 53.Kesharwani P, et al. Hyaluronic acid engineered nanomicelles loaded with 3,4-difluorobenzylidene curcumin for targeted killing of CD44+ stem-like pancreatic cancer cells. Biomacromolecules. 2015;16:3042–3053. doi: 10.1021/acs.biomac.5b00941. [DOI] [PubMed] [Google Scholar]
- 54.Kelava T, et al. Biological actions of drug solvents. Period Biol. 2011;113:311–320. [Google Scholar]
- 55.Pang X, et al. Hyaluronic acid-quercetin conjugate micelles: synthesis, characterization, in vitro and in vivo evaluation. Colloids Surf B Biointerfaces. 2014;123:778–786. doi: 10.1016/j.colsurfb.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, et al. Determination of paclitaxel in hyaluronic acid polymeric micelles in rat blood by protein precipitation-micelle breaking method: application to a pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;935:10–15. doi: 10.1016/j.jchromb.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 57.DeSantis CE, et al. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66:31–42. doi: 10.3322/caac.21320. [DOI] [PubMed] [Google Scholar]
- 58.Kesharwani P, et al. Carbon nanotube exploration in cancer cell lines. Drug Discov Today. 2012;17:1023–1030. doi: 10.1016/j.drudis.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Jain A, et al. Low density lipoproteins mediated nanoplatforms for cancer targeting. J Nanoparticle Res. 2013;15:1888. [Google Scholar]
- 60.Jin J, et al. Phototheranostics of CD44-positive cell populations in triple negative breast cancer. Sci Rep. 2016;6:27871. doi: 10.1038/srep27871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dent R, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–3334. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 62.Foulkes WD, et al. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 63.Zheng S, et al. Preparation of HIFU-triggered tumor-targeted hyaluronic acid micelles for controlled drug release and enhanced cellular uptake. Colloids Surf B Biointerfaces. 2016;143:27–36. doi: 10.1016/j.colsurfb.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 64.Zhong Y, et al. Hyaluronic acid-shelled acid-activatable paclitaxel prodrug micelles effectively target and treat CD44-overexpressing human breast tumor xenografts in vivo. Biomaterials. 2016;84:250–261. doi: 10.1016/j.biomaterials.2016.01.049. [DOI] [PubMed] [Google Scholar]
- 65.Li J, et al. Biological evaluation of redox-sensitive micelles based on hyaluronic acid-deoxycholic acid conjugates for tumor-specific delivery of paclitaxel. Int J Pharm. 2015;483:38–48. doi: 10.1016/j.ijpharm.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 66.Siegel R, et al. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 67.Wielenga VJ, et al. Expression of CD44 variant proteins in human colorectal cancer is related to tumor progression. Cancer Res. 1993;53:4754–4756. [PubMed] [Google Scholar]
- 68.Lee H, et al. Poly [lactic-co-(glycolic acid)]-grafted hyaluronic acid copolymer micelle nanoparticles for target-specific delivery of doxorubicin. Macromol Biosci. 2009;9:336–342. doi: 10.1002/mabi.200800229. [DOI] [PubMed] [Google Scholar]
- 69.Pitarresi G, et al. Self-assembled amphiphilic hyaluronic acid graft copolymers for targeted release of antitumoral drug. J Drug Target. 2010;18:264–276. doi: 10.3109/10611860903434027. [DOI] [PubMed] [Google Scholar]
- 70.Ishimoto T, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(−) and thereby promotes tumor growth. Cancer Cell. 2011;19:387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 71.Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17:313–319. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- 72.Thomas RG, et al. Paclitaxel loaded hyaluronic acid nanoparticles for targeted cancer therapy: In vitro and in vivo analysis. Int J Biol Macromol. 2015;72:510–518. doi: 10.1016/j.ijbiomac.2014.08.054. [DOI] [PubMed] [Google Scholar]
- 73.Zhou B, et al. Identification of the hyaluronan receptor for endocytosis (HARE) J Biol Chem. 2000;275:37733–37741. doi: 10.1074/jbc.M003030200. [DOI] [PubMed] [Google Scholar]
- 74.Wu J-L, et al. pH-responsive hyaluronic acid-based mixed micelles for the hepatoma-targeting delivery of doxorubicin. Int J Mol Sci. 2016;17:364. doi: 10.3390/ijms17040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stenzel MH. Bioconjugation using thiols: old chemistry rediscovered to connect polymers with nature’s building blocks. ACS Macro Lett. 2013;2:14–18. doi: 10.1021/mz3005814. [DOI] [PubMed] [Google Scholar]
- 76.Wang L, et al. Biomimic pH/reduction dual-sensitive reversibly cross-linked hyaluronic acid prodrug micelles for targeted intracellular drug delivery. Polymer (Guildf) 2015;76:237–244. [Google Scholar]
- 77.Jones DP, et al. Glutathione measurement in human plasma: Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta. 1998;275:175–184. doi: 10.1016/s0009-8981(98)00089-8. [DOI] [PubMed] [Google Scholar]
- 78.Han HS, et al. Bioreducible core-crosslinked hyaluronic acid micelle for targeted cancer therapy. J Control Release. 2015;200:158–166. doi: 10.1016/j.jconrel.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 79.Ikada Y, Tsuji H. Biodegradable polyesters for medical and ecological applications. Macromol Rapid Commun. 2000;21:117–132. [Google Scholar]
- 80.Shuai X, et al. Micellar carriers based on block copolymers of poly(ε-caprolactone) and poly(ethylene glycol) for doxorubicin delivery. J Control Release. 2004;98:415–426. doi: 10.1016/j.jconrel.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 81.Zhang H, et al. High intensity focused ultrasound-responsive release behavior of PLA-b-PEG copolymer micelles. J Control Release. 2009;139:31–39. doi: 10.1016/j.jconrel.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 82.Liang B, et al. High intensity focused ultrasound responsive metallo-supramolecular block copolymer micelles. Langmuir. 2014;30:9524–9532. doi: 10.1021/la500841x. [DOI] [PubMed] [Google Scholar]
- 83.Sun J, et al. The holistic 3M modality of drug delivery nanosystems for cancer therapy. Nanoscale. 2013;5:845. doi: 10.1039/c2nr32867d. [DOI] [PubMed] [Google Scholar]
- 84.Liu Y, et al. Folate and CD44 receptors dual-targeting hydrophobized hyaluronic acid paclitaxel-loaded polymeric micelles for overcoming multidrug resistance and improving tumor distribution. J Pharm Sci. 2014;103:1538–1547. doi: 10.1002/jps.23934. [DOI] [PubMed] [Google Scholar]
- 85.Nascimento TL, et al. Supramolecular organization and siRNA binding of hyaluronic acid-coated lipoplexes for targeted delivery to the CD44 receptor. Langmuir. 2015;31:11186–11194. doi: 10.1021/acs.langmuir.5b01979. [DOI] [PubMed] [Google Scholar]
- 86.Lokeshwar VB, et al. Targeting hyaluronic acid family for cancer chemoprevention and therapy. Adv Cancer Res. 2014;123:35–65. doi: 10.1016/B978-0-12-800092-2.00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y, et al. Recent advances in active hepatic targeting drug delivery system. Curr Drug Targets. 2014;15:573–599. doi: 10.2174/1389450115666140309232100. [DOI] [PubMed] [Google Scholar]
- 88.Zhang L, et al. Glycyrrhetinic acid-graft-hyaluronic acid conjugate as a carrier for synergistic targeted delivery of antitumor drugs. Int J Pharm. 2013;441:654–664. doi: 10.1016/j.ijpharm.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 89.Ahmed M, Narain R. Carbohydrate-based materials for targeted delivery of drugs and genes to the liver. Nanomedicine. 2015;10:2263–2288. doi: 10.2217/nnm.15.58. [DOI] [PubMed] [Google Scholar]
- 90.Liu Y, et al. Dual targeting folate-conjugated hyaluronic acid polymeric micelles for paclitaxel delivery. Int J Pharm. 2011;421:160–169. doi: 10.1016/j.ijpharm.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 91.Cheng D, et al. Multifunctional nanocarrier mediated co-delivery of doxorubicin and siRNA for synergistic enhancement of glioma apoptosis in rat. Biomaterials. 2012;33:1170–1179. doi: 10.1016/j.biomaterials.2011.10.057. [DOI] [PubMed] [Google Scholar]
- 92.Bush TL, et al. AMG 900, a small molecule inhibitor of aurora kinases, potentiates the activity of microtubule-targeting agents in human metastatic breast cancer models. Small Mol Ther. 2013;12:2356–2366. doi: 10.1158/1535-7163.MCT-12-1178. [DOI] [PubMed] [Google Scholar]
- 93.Xu J, et al. Aurora-A identifies early recurrence and poor prognosis and promises a potential therapeutic target in triple negative breast cancer. PLoS ONE. 2013;8:e56919. doi: 10.1371/journal.pone.0056919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Romanelli A, et al. Inhibiting Aurora kinases reduces tumor growth and suppresses tumor recurrence after chemotherapy in patient-derived triple-negative breast cancer xenografts. Mol Cancer Ther. 2012;11:2693–2703. doi: 10.1158/1535-7163.MCT-12-0441-T. [DOI] [PubMed] [Google Scholar]
- 95.Yin T, et al. Co-delivery of hydrophobic paclitaxel and hydrophilic AURKA specific siRNA by redox-sensitive micelles for effective treatment of breast cancer. Biomaterials. 2015;61:10–25. doi: 10.1016/j.biomaterials.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 96.Yang X, et al. Cluster of differentiation 44 targeted hyaluronic acid based nanoparticles for MDR1 siRNA delivery to overcome drug resistance in ovarian cancer. Pharm Res. 2015;32:2097–2109. doi: 10.1007/s11095-014-1602-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang X, et al. MDR1 siRNA loaded hyaluronic acid-based CD44 targeted nanoparticle systems circumvent paclitaxel resistance in ovarian cancer. Sci Rep. 2015;5:8509. doi: 10.1038/srep08509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ganesh S, et al. In vivo biodistribution of siRNA and cisplatin administered using CD44-targeted hyaluronic acid nanoparticles. J Control Release. 2013;172:699–706. doi: 10.1016/j.jconrel.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smejkalova D, et al. Selective in vitro anticancer effect of superparamagnetic iron oxide nanoparticles loaded in hyaluronan polymeric micelles. Biomacromolecules. 2014;15:4012–4020. doi: 10.1021/bm501065q. [DOI] [PubMed] [Google Scholar]
- 100.Qiu L, et al. Enhanced effect of pH-sensitive mixed copolymer micelles for overcoming multidrug resistance of doxorubicin. Biomaterials. 2014;35:9877–9887. doi: 10.1016/j.biomaterials.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 101.Alakhova DY, Kabanov AV. Pluronics and MDR reversal: an update. Mol Pharm. 2014;11:2566–2578. doi: 10.1021/mp500298q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jung H, Mok H. Mixed micelles for targeted and efficient doxorubicin delivery to multidrug-resistant breast cancer cells. Macromol Biosci. 2016;16:748–758. doi: 10.1002/mabi.201500381. [DOI] [PubMed] [Google Scholar]
- 103.Luca D, et al. Sur c vi a e l. 2011;2:755–63. [Google Scholar]
- 104.Cited R, City O, Data RU-A ( 12 ) United States Patent. 2003 doi: 10.1016/j.(73). [DOI]
- 105.Tsai, B., et al. (2005) (19) United States (12) 2005;1
- 106.Toleikis, P.M. and Jackson, J.K. (2006) (19) United States (12) 2006;1
- 107.Weiner WqK. Build Environ. 1989;24:265–77. [Google Scholar]
- 108.Chou, A. et al. (2014) City T, Wang S, Township J, Wei M, City Y. HA-g ZPCL, 2014
- 109.Amiji MM, Iyer AK. Multifunctional self-assembling polymeric nanosystems. 9173,840. US Pat No. 2015 Nov 3; n.d.