Abstract
Primary cutaneous CD8+ positive aggressive epidermotropic T- cell lymphoma is a rare and poorly characterized variant of cutaneous lymphoma still considered a provisional entity in the latest 2016 World Health Organization Classification of Cutaneous lymphomas. We sought to better characterize and provide diagnostic and therapeutic guidance of this rare cutaneous lymphoma. Thirty-four patients with a median age of 77 years (range 19 – 89 years) presented primarily with extensive annular necrotic plaques or tumor lesions with frequent mucous membrane involvement. The 5-year survival was 32% with a median survival of 12 months. A subset of 17 patients had a prodrome of chronic patches prior to the development of aggressive ulcerative lesions. We identified cases with lack of CD8 or αβ T-cell receptor expression yet with similar clinical and pathological presentation. Allogeneic stem cell transplantation provided partial or complete remissions in 5/6 patients. We recommend the term primary cutaneous aggressive epidermotropic cytotoxic T-cell lymphoma as this more broad designation better describes this clinical-pathologic presentation which allows the inclusion of cases with CD8 negative and/or αβ/γδ T-cell receptor chain double positive or double negative expression. We have identified early skin signs of chronic patch/plaque lesions that are often misdiagnosed as eczema, psoriasis, or MF. Our experience confirms the poor prognosis of this entity and highlights the inefficacy of our standard therapies with the exception of allogeneic stem cell transplantation in selected cases.
INTRODUCTION
Primary cutaneous CD8 positive aggressive epidermotropic T-cell lymphoma remains a provisional entity in the latest World Health Organization 2016 classification of cutaneous lymphomas (1). In part the problem with CD8 positive aggressive epidermotropic T-cell lymphoma is that they have been poorly characterized to date due to the rarity of the condition. Historically, this condition was known in the dermatology literature as generalized pagetoid reticulosis or Ketron-Goodman disease and was characterized by rapidly evolving and extensively ulcerated annular plaques associated with a poor prognosis. The histopathology has been reported to be characteristic, with an infiltrate of monomorphous medium sized atypical lymphocytes involving the epidermal thickness in a so-called pagetoid pattern. While clinically this condition was known to result in widespread ulcerations from epidermal necrosis, the cytotoxic phenotype with CD8 expression was first reported by Agnarsson et al in 1990, and the term CD8 positive aggressive epidermotropic T-cell lymphoma was first coined by Berti et al in 1999 (2, 3). We have observed cases with similar clinical-pathologic features, cytotoxicity and aggressive course without CD8 expression. Our goal is to redefine this entity using the more broad and inclusive term primary cutaneous aggressive epidermotropic cytotoxic T-cell lymphoma (PCAECyTCL). Herein we present the largest series of primary cutaneous aggressive epidermotropic cytotoxic T-cell lymphoma reported to date, collected from several cutaneous lymphoma centers in the United States.
MATERIALS AND METHODS
A group of pathologists and dermatologists from some of the largest cutaneous lymphoma centers in the United States met on two occasions in Chicago to review each institutional experience including the clinical and pathological material of primary cutaneous aggressive epidermotropic cytotoxic T-cell lymphoma. Approval from the Institutional Review Board at Northwestern University encompassing all centers was obtained. Entering criteria included a clinical presentation with patches, plaques or nodules with erosive or necrotic features covering more than 1% of the body surface area. Cases with localized pagetoid reticulosis (Woringer-Kolopp disease) were excluded. The cases included were characterized by 1) a pagetoid pattern of T-cells with expression of at least one pan-T-cell marker 2) positivity for CD8 and BF1 (αβ T-cell receptor heterodimer) and 3) negativity for the γδ T-cell receptor heterodimer marker (clone γ3.20, Thermo/Fisher Scientific, Waltham, MA). However, these criteria were revised when we encountered cases that fulfilled clinicopathologic criteria but with variable CD8 and/or T-cell receptor immunophenotype. An extensive immunohistochemistry panel of monoclonal antibodies against CD2, CD3, CD4, CD5, CD7, CD8, CD20, CD30, CD45RA, CD56, T-cell intracellular antigen-1 and granzyme B was performed in most cases. In addition Epstein-Barr virus ribonucleic acid expression was evaluated using Epstein-Barr encoding region in situ hybridization and T-cell receptor clonality analysis with polymerase chain reaction based methods using BIOMED-2 protocol was reported when available from skin, blood, nodal and bone marrow biopsy samples.
Electronic medical records were reviewed and clinical, imaging and laboratory parameters along with clinical photographs when available were presented to the group. Clinical data including gender, age, race, nature, extent and duration of the lesions, constitutional symptoms, past medical history, results of staging bone marrow biopsy and imaging tests, response to therapies, outcome and duration of follow up were obtained. The original histopathological slides including immunohistochemical markers were reviewed by the group and a consensus diagnosis was rendered. Skin biopsies from the 30 selected patients were reviewed. Several parameters, including the distribution of the infiltrate within the epidermal, dermal and subcutaneous compartments, distribution of the intraepidermal lymphocytes, cell size, adnexotropic features (folliculotropism and syringotropism), presence or absence of epidermal necrosis, interface reaction, ulceration, hemorrhage, angiocentricity or vasculitis were assessed by the group on a multiheaded microscope. Other notable histological findings such as presence of other inflammatory cells (eosinophils, neutrophils, plasma cells, histiocytes), granuloma formation, mucin deposits, karyorrhexis or hemophagocytosis were documented.
Descriptive statistics including median (with range) and counts (with percentages) were determined and compared using U Mann Whitney and Fisher’s Exact tests, respectively. Five-year survival rate was performed using Kaplan-Meier method. Categorical clinical and pathological characteristics were compared using log-rank tests to overall outcome and survival rates. Survival analysis was performed from date of diagnosis. As this was a retrospective analysis, data were missing for some cases. All analyses were done on the available data. All tests were performed using SPSS statistics software (Chicago, IL) with a type I error rate of 5%. P-values less than 0.05 were considered indicative of a trend.
RESULTS
Clinical Data
Upon a consensus review of a total of 52 cases, 30 were deemed to fulfill the diagnostic criteria and confirmed by the group as primary cutaneous aggressive epidermotropic cytotoxic T-cell lymphoma and 4 additional cases were added to the series over the ensuing months (Table 1). One case had been previously published as a case report (4). All cases were collected between 1995 and 2015. Most of the cases excluded were redefined after reviewing clinical and histological parameters by the group as CD8+ mycosis fungoides, pagetoid reticulosis or dermal CD8+ T cell lymphomas without marked epidermotropism or pagetoid features.
Table 1.
Clinical summary of the cohort.
| Case | Age/Gender/Race | Time before diagnosis (mo) | Clinical presentation | BSA | TNM at presentation | Treatment | Outcome | Follow up (mo) |
|---|---|---|---|---|---|---|---|---|
| 1 | 60/M/B | n/a | Plaques | 12 | T2N0M0 | XRT, IFN | AWOD | 144 |
| 2 | 82/M/W | 1 | Patch, plaque, tumors | n/a | n/a | n/a | n/a | n/a |
| 3 | 73/M/U | 12 | Plaque | 75 | T3N1M0 | MTX | n/a | n/a |
| 4 | 19/F/H | 6 | Patch, plaque | 80 | T3N0M0 | NM, TSEBT, Romi, EPOCH, Gem, UCTH1, Bendamustine, HSCT | AWOD | 46 |
| 5 | 28/M/H | 3 | Psoriasiform dermatitis | 36 | T3N0M0 | TSEBT, BXT, Romi, Liposomal doxorubicin, vorinostat, PTX, Brentuximab, CHOP, EPOCH, Gem, Denileukin Diftitox, HSCT | AWD | 168 |
| 6 | 46/F/W | 3 | Ulcerated nodule | <10 | T2N0M0 | Phototherapy, TSEBT, Liposomal doxorubicin, PTX, Brentuximab, Gem, Bendamustine, HSCT | AWD | 24 |
| 7 | 89/M/W | 12 | Psoriasiform dermatitis | >20 | T2N0M0 | ECP, Chemo | Dead | 12 |
| 8 | 78/F/H | 2 | Ulcerated plaque | 1 | T1N0M0 | XRT | AWD | n/a |
| 9 | 82/M/W | 3 | Plaques | 0.45 | T2N0M0 | Gem, Liposomal doxorubicin, BXT, NM | AWD | 18 |
| 10 | 81/M/B | 12 | Hand eczema | T3N0M0 | Gem | Dead | n/a | |
| 11 | 42/F/W | 72 | Eczema | 80 | n/a | ECP, PUVA | Dead | 36 |
| 12 | 65/M/B | (years) | Eczema | 35 | T3N0M0 | n/a | Dead | n/a |
| 13 | 87/F/W | 36 | Erythematous rash/ulcerated plaques | 30 | T3N0M0 | TSEBT, NM, BXT, Vorinostat | Dead | 15 |
| 14 | 83/M/U | 12 | Crusted/ulcerated plaques | 5 | T3N1M0 | CHOP, XRT | Dead | 7 |
| 15 | 76/M/W | 36 | Psoriasiform dermatitis | 94 | T3N0M1 | Liposomal doxorubicin, BXT, Romi, PTX | Dead | 4 |
| 16 | 85/M/U | 5 | Plaques/ulcerated tumors | 45 | T3N1M0 | None (died before able to initiate) | Dead | <1 |
| 17 | 80/F/W | 3 | Eczema/ulcerated tumors | 5 | T3N0M0 | Brentuximab, Romi, PTX | AWOD | 9 |
| 18 | 80/F/U | 11 | Psoriasiform dermatitis | 35 | T3N0M0 | TSEBT, BXT, Romi | Dead | 7 |
| 19 | 69/M/U | 120 | Patches and tumors | <5 | T3N0M1 | XRT | Dead | 24 |
| 20 | 86/M/W | 3 | Plaques and tumors | n/a | T3N0M0 | Palliative XRT, Bortezomib | Dead | n/a |
| 21 | 63/M/W | 84 | Psoriasiform dermatitis | 50 | T3N0M0 | IFN, CHOP, Phototherapy | Dead | n/a |
| 22 | 70/M/B | 10 | Spreading nodules | 80 | T3N0M0 | BXT, Denileukin Diftitox, TSEBT | Dead | 4 |
| 234 | 80/M/W | n/a | Erythematous and scaly patch | n/a | T3N0M0 | BXT, TSEBT, NM | n/a | n/a |
| 24 | 80/M/W | 1.5 | Psoriasiform dermatitis, tumors | 5 | T3N1M0 | Liposomal doxorubicin, Gem | Dead | 12 |
| 25 | 31/M/H | 24 | Necrotic ulcerations | 50 | T3N1M0 | Hyper-CVAD, BXT, PUVA, HSCT | AWD | 84 |
| 26 | 72/M/W | 5 | Ulcerated plaque, tumors | 35 | T3N0M0 | NM, phototherapy, XRT, BXT, Vorinostat, Gem, GND, CMED, Hydroxyurea, Denileukin diftitox | Dead | 72 |
| 27 | 87/M/A | 6 | Ulcerated plaques | 20 | T3N0M0 | Gem, CHOP, MTX, PTX, Liposomal doxorubicin, Romi, BXT | AWD | 12 |
| 28 | 59/F/W | 8 | Psoriasiform dermatitis | 15 | T3N0M0 | Phototherapy | AWD | 8 |
| 29 | 63/M/W | 60 | Patches, tumor | 20 | T3N0M0 | CHOEP, TSEBT, HSCT | Dead | 10 |
| 30 | 27/M/B | 18 | Psoriasiform dermatitis | 70 | T3N0M0 | MTX | AWD | n/a |
| 31 | 88/M/W | 12 | Plaques | 20 | n/a | XRT, phototherapy | AWD | 36 |
| 32 | 85/M/W | 6 | Patch, plaque, tumors | 12 | n/a | XRT, CEP | Dead | 12 |
| 33 | 21/F/W | 6 | Ulcerated plaque | n/a | T1N0M0 | EPOCH, Bendamustine, DHAP, SMILE, Gem, PTX, Romi, XRT, HSCT | AWOD | 16 |
| 34 | 86/M/W | 108 | Patches and plaques | 20 | T3N0M0 | NB-UVB, Acitretin, TSEBT | AWD | 1 |
A: Asian; AWD: alive with disease; AWOD: alive without disease; B: Black; BSA: body surface area; BXT: bexarotene; CEP: cisplatin, epirubicin, paclitaxel; CHOEP: cyclophosphamide, doxorubicin, etoposide, vincristine, prednisone; CHOP: cyclophosphamide, adriamycin, vincristine, prednisone; CMED: cyclophosphamide, methotrexate, etoposide, dexamethasone; DHAP: dexamethasone, cytarabine, cisplatin; ECP: extracorporeal photophoresis; EPOCH: Etoposide, prednisone, Vincristine, cyclophosphamide, doxorrubicine; F: female; Gem: Gemcitabine; GND: Gemcitabine, navelbine, doxorubicin; H: Hispanic; HSCT: hematopoetic stem cell transplant; Hyper-CVAD: cyclophosphamide, vincristine, doxorubicine hydrochloride, dexamethasone; IFN: interferon; M: male; MTX: methotrexate; n\a: non-aplicable or available; NM: Nitrogen mustard; PTX: pralatexate; PUVA: psoralen ultraviolet light A; Romi: romidepsin; SMILE: dexamethasone, methotrexate, ifosfamide, l-asparaginase, etoposide; SMILE: dexamethasone, methotrexate, ifosfamide, l-asparaginase, etoposide; TSEBT: Total skin electronbeam; UCTH1: anti-human CD3; U: Unknown; XRT: radiation; W: white.
The cohort consisted of 34 patients (25 male, 9 female) with a median age of 77 years (range 19–89 years) including 19 White, 5 Blacks, 4 Hispanic, 1 Asian and 5 of unknown race. The median duration of the lesions at the time of presentation was 10 months, ranging from 1 to 120. In 14 of 31 subjects the onset of symptoms was considered acute appearing within 6 months from the time of presentation. Seventeen patients had a chronic course longer than 6 months with poorly characterized preceding skin lesions. There was no survival difference from the time of diagnosis between those with an acute versus chronic course (p=.09). The skin lesions at presentation were reported as patches or plaques in 24 patients, tumors in 2 patients (Figure 1a, Case 24) and a combination of both in 7 patients. Ulceration was noted at presentation in 10 cases and 20 additional cases evolved into ulcerated plaques overtime. Thirteen patients carried an initial diagnosis of “mycosis fungoides”, whereas many other cases were initially diagnosed with a benign condition including an eczematous process, contact dermatitis, viral or drug eruption, psoriasis (11 cases), lupus erythematosus (2 cases), erythema multiforme or ulcer. Two patients presented with acute coalescing necrotic macules resembling extensive pityriasis lichenoides acuta (Mucha-Habermann disease) (Figure 1b, Case 27). We also reviewed skin biopsies from two patients with the preceding non-specific skin presentations. In both cases rare scattered intraepidermal atypical lymphocytes were noted accompanied with acanthosis, hyperkeratosis and rare necrotic keratinocytes. Using the TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sézary syndrome, multifocal or generalized skin involvement at presentation (T3) was reported in 24 patients (5). Two patients presented with a solitary lesion (T1) and 4 patients with localized involvement in one anatomic region (T2). Disease progression requiring systemic therapy was reported in all patients except in one with limited disease. The skin lesions were for the most part annular and erythematous with peripheral erosions and ulceration often surmounted with a hemorrhagic-necrotic crust (Figure 2a–c, Case 25). The ulcerated patches tended to become more generalized and extensive in advanced stages (Figure 2d,e, Case 26). The distribution of the lesions was variable with involvement of the upper and/or lower extremities in 29 patients, torso in 23 and head/neck in 15 patients. Five patients developed ulcers involving the oral cavity and 6 patients involving genital or perigenital skin (Figure 1c, Case 23 and 2a, Case 25). Constitutional symptoms were overall mild with a few patients reporting weight loss (6), low grade fever (3) and asthenia (3). Past medical history was significant for 3 patients with solid organ cancer (uterine, prostate, melanoma). One of these patients also had Parkinson disease, and one patient had a remote history of Hodgkin lymphoma in remission. Only one patient was immunosuppressed post-lung transplant and one patient had an unconfirmed diagnosis of bullous pemphigoid. Serum lactate dehydrogenase levels were increased at the time of presentation (>200 U/L) in 26% (5/19) of the patients. Four patients had abnormal peripheral blood results by flow cytometry including one patient with CD8+/CD26+ leukemia of 75,000 cells/ml. The same patient was the only one (1/11) with positive bone marrow biopsy. Small palpable lymphadenopathy was noted in 6 cases, but none of them were considered clinically significant for nodal biopsy. Clinical or pathological signs of hemophagocytosis or hepatosplenomegaly were not reported in any of the cases.
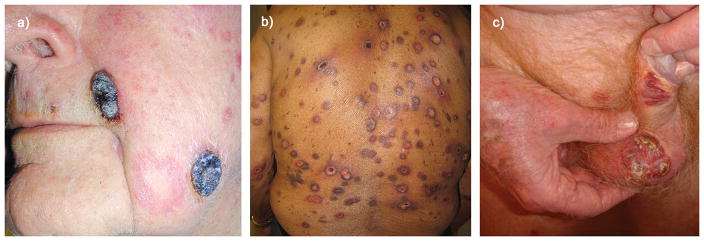
Figure 1. Clinical presentation of PCAECyTCL in three different patients.
a) Tumor presentation with exophytic hemorrhagic and necrotic nodules resembling pyogenic granuloma (Case 24). b) Extensive targetoid necrotic macules and plaques resembling severe pityriasis lichenoides acuta or erythema multiforme (Case 27). c) Ulcerated plaques involving genitalia (Case 23).
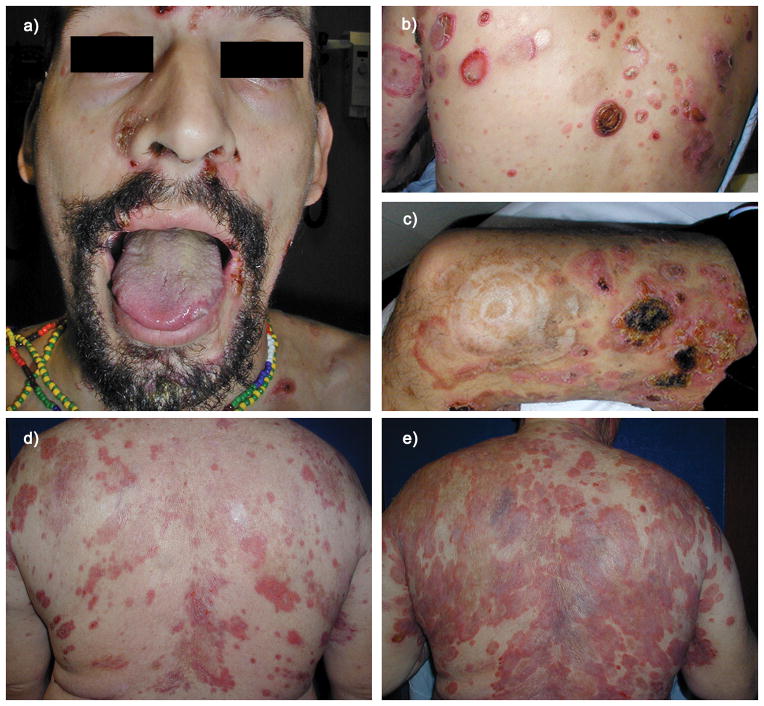
Figure 2. Clinical presentation of PCAECyTCL in two different patients.
a) Oral and nasal ulcerated macules (Case 25). b) Extensive annular erosive plaques some with hemorrhagic features (Case 25). c) Annular and arcuate ulcerated plaques and scars at sites of resolved lesions (Case 25). d) Annular erythematous plaques at presentation (Case 26). e) Same patient as 2D now with extensive erosive confluent plaques a few months later (Case 26).
Results from imaging studies at presentation were available for 25 patients and were reported positive in 11 patients, most commonly demonstrating positron emission tomography avid cutaneous or subcutaneous uptake with only 1 patient with nodal involvement and 1 patient with right adrenal involvement.
Follow up information was available for 31 patients with a median follow up time of 12 months (range 1 – 168 months). Seventeen patients (54%) died during the follow up period related to disease progression. Four patients developed extracutaneous disease involving lungs (2) and adrenal glands (2). None of the patients developed hemophagocytic syndrome. At the last follow up, 12 patients were known to be alive with disease, three alive without disease. The 5-year overall survival rate was 32% with a median overall survival of 12 months.
A median of 3 therapies (range 1–13), including skin-directed and systemic regimens, were administered to the cohort. Eight of the 20 patients treated with various chemotherapies were alive at the end of the study. Ten patients received localized radiation therapy and 8 patients total skin electronbeam therapy. Six patients were treated with allogeneic hematopoietic stem cell transplantation and 5 of them were alive at the end of the study including 2 in complete remission and 3 with recurrent disease albeit mostly limited to a few sporadic lesions. One patient developed extensive extracutaneous disease shortly after allogeneic hematopoietic stem cell transplantation while on immunosuppressive medication (detailed summary of therapies is presented in Table 2).
Table 2.
Clinical outcomes of radiation and systemic therapies.
| Therapeutic agents | Number of patients who received the agent (%) | Number of patients who achieved any response including CR, PR* | Significance regarding survival outcome, p-value |
|---|---|---|---|
| Etoposide | 6 (21) | 2 | .59 |
| Gemcitabine | 9 (30) | 3 | .13 |
| Local radiation | 10 (33) | 5 | .60 |
| TSEB** | 8 (26) | 6 | .56 |
| Bexarotene (oral) | 9 (30) | 1 | .56 |
| Romidepsin | 6 (21) | 1 | .16 |
| Liposomal doxorubicin | 6 (21) | 2 | .24 |
| Allogeneic stem cell | 6 (21) | 5 | .06 |
| transplant |
CR: complete remission; PR: partial response; TSEB**: total skin electron beam. None of the treatments were significantly associated with a good outcome.
Pathology data
Thirty-four biopsies were assessed
The pattern of the lymphoid infiltrate was pagetoid in all cases with scattered atypical lymphocytes involving the entire epidermal thickness in 20 biopsies, while in 14 biopsies the atypical lymphocytes were predominantly confined to the lower half of the epidermis (Figure 3a–d Case 25, Case 34 and Case 29). The latter pattern was commonly associated with an atrophic epidermis. Infiltration of cutaneous adnexa by the lymphoma cells was common, but eccrine glands or follicular units were absent from some skin biopsies (Figure 3e-f, Case 26). Syringotropism of the eccrine glands without hyperplastic changes was noted in 18/30 biopsies and folliculotropism in 18/22. However, follicular mucinosis or destruction of the follicular unit by the tumor cells as commonly seen in folliculotropic MF were not observed in any case. In addition to the intraepithelial infiltrate, 9 biopsies had a tumoral dermal component and in 6 additional biopsies the infiltrate extended beyond the reticular dermis into the subcutaneous tissue (Figure 3e). Focal (12/31) or extensive and confluent (8/31) keratinocyte necrosis was noted in 64% of the biopsies with frank ulceration in 7 biopsies. However other biopsies (14/34) showed no signs of epidermal necrosis regardless of lymphoid density. Hence necrosis, hemorrhage or other histological signs of cytotoxicity may not be observed in some skin biopsies of early evolving lesions. Intraepidermal aggregates of atypical lymphocytes resembling Pautrier’s microabscesses were observed in 16/34, more commonly seen in biopsies with high intraepidermal lymphoid density, rather than the scattered microaggregates usually seen in MF. Mild acanthosis was noted in 15 biopsies, but none of the samples had the verrucous hyperplasia so characteristic of pagetoid reticulosis. While upper dermal extravasation of erythrocytes was common (20/34) often with subtle infiltration of small vessel walls (3/34), fibrinoid vascular necrosis was not noted. However, frank angiocentric or angiodestructive features were not identified in any of the cases. Likewise, modest and focal neurotropism was observed in 5/34 cases. Only one of these biopsies was associated with CD56 (neural cell adhesion molecule) expression and none of them had clinical signs of neuropathy. A minor component of eosinophils in the infiltrate was observed in (13/34) including 9 with folliculotropism/syringotropism. Few neutrophils (10/34) or plasma cells (2/34) were noted within the infiltrate in rare biopsies.
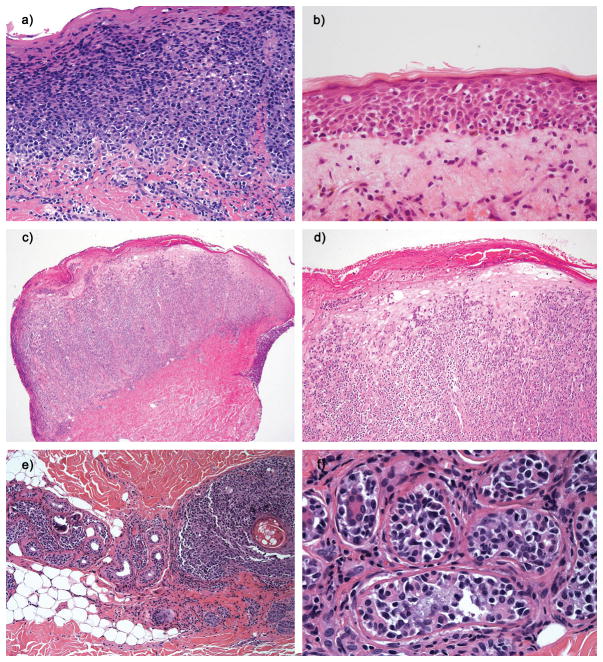
Figure 3. Histopathological features of PCAECyTCL.
a) H&E stains showing extensive full-thickness pagetoid exocytosis of medium sized lymphocytes without significant keratinocytes necrosis or dermal involvement (Case 25). b) Atrophic epidermis with pagetoid intraepidermal atypical small/medium lymphocytes mostly involving the lower levels of the epidermis. Occasional necrotic keratinocytes are noted (Case 34). c) Tumor lesion with a predominantly dermal infiltrate (Case 29). d) Same as C in higher magnification showing extensive pagetoid exocytosis in addition to a dense dermal infiltrate (Case 29). e) Marked lymphoid infiltration of eccrine coils on the left side and pilosebaceous unit on the right side (Case 26). f) High power of image e demonstrating atypical small lymphocytes in the eccrine coil (Case 26).
The cytological detail of these lymphomas was rather monomorphous and consisted predominantly of medium-sized lymphocytes (24/34). The tumor cells displayed oval or slightly indented nuclei with scant cytoplasm with indistinct cell boundaries (Figure 3f). A predominantly large cell infiltrate was identified in 3 biopsies.
Immunophenotypic and molecular data
All 34 skin biopsies analyzed with immunohistochemistry were CD3 positive and 34/34 were CD8 positive while 7 biopsies had a CD4−/CD8− double negative phenotype and one of the CD8+ biopsies also showed weak CD4 co-expression. We noted CD8 expression to be of variable intensity from case to case (Figure 4a, Case 30). βF1 was positive in 27/33 biopsies. The six βF1 negative biopsies were also negative for the γδ immunomarker. In addition, two biopsies expressed both, the γδ marker (γ3.20) and the αβ (βF1) T–cell receptor heterodimers (Figure 4c–f, Case 27). Four of the 30 biopsies tested had partial CD30 positivity, including the 2 biopsies with predominance of large cells. CD2 was negative in 56% (13/23) of the biopsies. CD56 was positive in 12% (4/34) of the biopsies and CD5 was positive in 33% (11/33) of the biopsies evaluated. CD45RA was expressed in 61% (16/26) of the biopsies and CD7 was positive in 79% (23/31) of the biopsies. Co-expression of CD7 and CD45RA was noted in 12/26 biopsies. Expression of at least one cytotoxic marker including T-cell intracellular antigen-1 (31/32) and granzyme-B (22/29) was noted in all biopsies with one exception (Figure 4b, Case 30). Epstein-Barr encoding region in situ hybridization was negative in all 31 biopsies evaluated. T-cell receptor clonality by polymerase chain reaction methods was detected in the skin of 57% (4/7) of the biopsies available for evaluation. T-cell clonality was also detected in the blood of two subjects. Only one case presented with the same clone in the skin and blood by T-cell receptor, which also had a small population by morphology (6%).
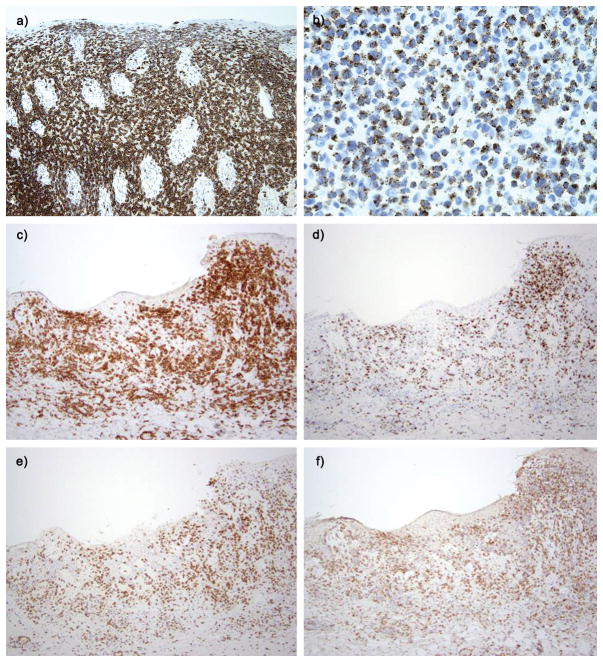
Figure 4. Histopathological features of PCAECyTCL.
Immunohistochemistries of case 30 illustrate in 3a with strong CD8 expression (a) and TIA-1 (b). Immunochemistries of case 27 illustrated in figure 1b including CD8 (c), granzyme B (d), and coexpression of γδ marker (e) and βF1 (f).
Clinical or Pathologic Parameters Associated with Survival Outcome (Table 4)
Table 4.
Clinical or pathologic parameters associated with survival outcome.
| Parameter | Cases (dead/total cases ) | p-value | |
|---|---|---|---|
| Gender | Male 10/16 vs. female 3/8 | .50 | |
| Race | Caucasian 8/14 vs. others 1/6 | .21 | |
| Length of disease before diagnosis | 6m 9/12 vs. >6m 4/11 | .09 | |
| Ulcers at presentation | Present 7/15 vs. absent 6/9 | .69 | |
| Constitutional symptoms | Present 5/8 vs. absent 5/11 | .36 | |
| Stage | T1/T2 1/5 vs. T3 10/16 | .11 | |
| Total number of therapies | 1–3 therapies 8/11 vs. 4 – 13 therapies 4/12 | .14 | |
| Abnormal LDH | Abnormal 3/3 vs. normal 5/10 | .16 | |
| Mucosal membrane involvement | present 2/5 vs. absent 11/19 | .51 | |
| Tumors at diagnosis | Present 4/4 vs. absent 9/20 | .04 | |
| Tumors at any time | Present 10/15 vs. absent 3/9 | .17 | |
| Extracutaneous disease | Present 3/4 vs. absent 7/15 | .31 | |
| Large cell (s/m vs Large) | S/m 9/14 vs. large 4/10 | .30 | |
| CD2 expression | Positive 4/7 vs. negative 3/10 | .14 | |
| CD5 expression | Positive 4/10 vs. negative 8/13 | .37 | |
| CD7 expression | Positive 11/19 vs. negative 1/4 | .69 | |
| CD45RA expression | Positive 7/11 vs. negative 3/6 | .77 | |
| CD45RA/CD7 expression | Positive 6/10 vs. negative 7/13 | .85 | |
| CD4-CD8- expression | Positive 3/5 vs. negative 10/19 | .42 | |
| CD30 expression | Positive 1/3 vs. negative 11/20 | .36 | |
| Granzyme B expression | Positive 10/18 vs. negative | 3/5 | .95 |
| CD56 expression | Positive 2/2 vs. negative 11/22 | .23 | |
There was no statistically significant differences in the survival outcome between genders, race, length of disease (>6 months vs. <6 months), clinical ulcers, or constitutional symptoms at presentation, skin stage T1/ T2 versus T3, number of therapies used, high serum lactate dehydrogenase, or mucosal membrane involvement. A significant worse prognosis was noted in patients presenting with tumors at diagnosis. Extracutaneous involvement did not reach statistical significance, but there were only 4 patients with it and one of them was alive after allogeneic hematopoietic stem cell transplantation. No statistically significance difference in survival was found associated with large cell (small/medium vs. large) or immunophenotypes CD2, CD5, CD7, CD45RA, CD45RA/CD7, CD4−/CD8−, CD30, granzyme B and CD56. None of the treatment modalities showed significant survival advantage (Table 2). Allogeneic hematopoietic stem cell transplantation resulted in prolonged partial or complete remissions in 5 of 6 patients but did not reach statistical significance.
DISCUSSION
We have reviewed our experience with a rare and aggressive subtype of cutaneous T-cell lymphoma still considered a provisional entity by the latest World Health Organization classification of cutaneous lymphomas. CD8 positive aggressive epidermotropic T-cell lymphoma has been defined clinically by the abrupt presentation of extensive annular plaques with erosive features and ulceration (6, 7). This observation reflects the cytotoxic nature of the lymphoma cells which are embedded primarily in the intraepithelial compartments including epidermis and skin adnexa. The lymphoma cells contain cytotoxic granules, resulting in epidermal necrosis upon activation. The factors that trigger the cytotoxic events are poorly understood, but cases with marked epidermotropism of lymphoma cells without necrotic keratinocytes contrast with other cases with fewer lymphocytes yet with massive keratinocyte necrosis or ulceration. At least in some reported cases, phototherapy and interferon may have coincided with this activation of cytotoxic events (8). Angioinvasion and angiodestructive features have been occasionally reported in CD8 positive aggressive epidermotropic T-cell lymphoma (7, 9). While subtle involvement of dermal vasculature by the lymphoma cells was observed in a few cases, ulceration seems to be the direct result of epithelial necrosis from cytotoxic lymphocytes rather than a consequence of vaso-occlusion or angiodestruction as seen in natural killer-T cell lymphomas associated with Epstein-Barr virus infection. This pathomechanism of massive epidermal necrosis triggered by a clonal expansion of cytotoxic T-cells is also shared by pityriasis lichenoides acuta or Mucha-Habermann disease. Hence, it comes as no surprise that the febrile ulcero-necrotic variant of Mucha-Habermann can pose a diagnostic dilemma, as observed in 2 of our cases. By definition, extensive epidermotropism is a diagnostic requirement, but in some cases the epidermal lymphocytes remained localized in the lower epidermal layers. Furthermore, the burden of tumor cells may be dermal in thick plaque or tumor lesions with pagetoid extension into the surmounting epidermis.
As reported by Robson et al, despite extensive adnexotropism, clinical evidence of folliculotropism including follicular based papules (keratosis pilaris, lichen spinulosus, milia or cystic lesions) or alopecia mucinosa were not observed in our series (7). In addition, destruction of adnexal structures with resulting granulomatous reaction or mucinous degeneration as seen in folliculotropic mycosis fungoides do not seem to occur in primary cutaneous aggressive epidermotropic cytotoxic T-cell lymphoma.
Unlike other cytotoxic lymphomas such as γδ T cell lymphomas that are often associated with immune dysregulation or natural killer-T cell lymphomas associated with epstein-barr virus infection, we have not identified any significant co-morbidities or predisposing factors associated with primary cutaneous aggressive epidermotropic cytotoxic T-cell lymphoma (10). However, preceding skin signs of poorly defined papulo-squamous or eczematous dermatitis were observed in a several patients. The clinical impression of these preceding lesions varied from psoriasis to eczema or mycosis fungoides. We had the opportunity to review skin biopsies of preceding lesions of two cases which showed psoriasiform acanthosis with subtle scattered intraepidermal atypical lymphocytes in one case and folliculotropism and syringotropism with eosinophils in another case resembling adnexotropic mycosis fungoides. Previous reports of similar epidermotropic cytotoxic lymphomas initially misinterpreted as “ulcerative psoriasis” also emphasized the fact that a lymphoid-rich skin biopsy should be a warning sign not compatible with psoriasis vulgaris (11). In addition, we noted scattered necrotic keratinocytes in these early lesion biopsies, a finding rarely seen in psoriasis or mycosis fungoides.
As initially reported by the European group, the World Health Organization defines CD8 positive aggressive epidermotropic T-cell lymphoma by the expression of CD8. However, we have observed cases with CD4/CD8 double negativity that otherwise fulfill clinical and pathological characteristics of this entity. Likewise, CD45RA, a marker commonly expressed in CD8 positive aggressive epidermotropic T-cell lymphoma, was negative or partially positive in many cases and hence not a reliable diagnostic marker. The variable CD45RA expression is not surprising since the marker is reversible with stronger expression in resting and naïve lymphocytes when antigenic stimulation is low (12). In addition, we observed variable expression of the T–cell receptor heterodimer markers. A small subset of cutaneous lymphomas with similar clinicopathological presentation expressing only γδ T–cell receptor heterodimer with αβ negativity were reviewed and excluded for this study. However, a few other cases with αβ/γδ double positive or double negative expression were included. This unexpected variable expression of T–cell receptor heterodimers ranging from αβ/γδ T–cell receptor double positivity to double negativity has also been reported in natural killer-T-cell lymphomas (13). This observation may not be entirely surprising since the γδ immunomarkers for paraffin embedded tissue were not widely available when Berti et al published their initial case series (14).
A recent publication by Merrill et al highlighted aggressive primary cutaneous gamma delta T-cell lymphoma presenting with pagetoid epidermotropism.(15) We considered this diagnostic possibility for the two cases expressing the gamma T-cell receptor in addition to the beta T-cell receptor marker (cases 22 and 27). However, we felt the clinical and pathological findings of these cases was most consistent with the rest of the primary cutaneous aggressive epidermotropic cytotoxic T-cell lymphoma cohort and as previously mentioned variable expression of alpha/beta and gamma/delta markers is occasionally observed in other cytototxic lymphomas. Based on our observation, we recommend the term primary cutaneous aggressive epidermotropic cytotoxic T-cell lymphoma, to include not only the previously described CD8+/ T–cell receptor -β+ cases but the additional CD4/CD8 double negative cases and/or cases with variable αβ/γδ heterodimer expression, as long as at least one T cell marker and one marker of cytotoxicity (i.e. T-cell intracellular antigen-1, perforin or granzyme-B) is expressed.
Our cohort confirms the poor prognosis of primary cutaneous aggressive epidermotropic cytotoxic T-cell lymphoma with rapid disease progression, poor response to chemotherapy and death often associated with complications of extensive epidermal necrosis and only rarely nodal or other extracutaneous involvement. In contrast to other cytotoxic lymphomas, hemophagocytic lymphohistiocytosis was not observed in any of our cases. We found one case report of primary cutaneous aggressive epidermotropic cytotoxic T-cell lymphoma complicated with hemophagocytic lymphohistiocytosis in the literature, but the diagnosis of this human immunodeficiency virus positive patient with epstein-barr virus expression in the intraepidermal lymphoid cells is questionable (16).
Besides the rare and equally aggressive epidermotropic variants of cutaneous γδ T-cell lymphomas, the differential diagnosis of primary cutaneous aggressive epidermotropic cytotoxic T-cell lymphoma includes CD8 positive mycosis fungoides, localized pagetoid reticulosis (Woringer-Kolopp disease) and type D lymphomatoid papulosis. These three indolent conditions can occasionally present with a similar complete or partial CD45RA+/CD7+/CD8+ phenotype profile (7, 12). The histopathology of mycosis fungoides tends to be less pagetoid with more pleomorphic and convoluted nuclei and most importantly without necrotic keratinocytes. Intraepidermal aggregates of lymphocytes resembling Pautrier microabscesses were a common finding and hence may not help distinguish from mycosis fungoides. Localized pagetoid reticulosis (Woringer-Kolopp disease) and type D lymphomatoid papulosis can have similar biopsy findings including epidermal necrosis, but usually the clinical presentation does not pose a diagnostic dilemma. Nevertheless, one of our patients initially presented with a single lesion and the diagnosis of Woringer-Kolopp disease was considered, but extensive patches appeared shortly after.
Based on our experience, we feel that primary cutaneous aggressive epidermotropic cytotoxic T-cell lymphoma should be defined clinically by the rapid onset of erosive or ulcerated plaques with or without preceding skin lesions and should not be limited to immunophenotypic expression of CD8 or the αβ T–cell receptor heterodimer, but rather the identification of an atypical pagetoid T-cell infiltrate with cytotoxic granules and absence of CD4 expression. Molecular studies should demonstrate dominant T–cell receptor sequences. We also bring attention to the yet poorly defined preceding skin lesions reported in several of our patients and often misdiagnosed as psoriasis, eczema or mycosis fungoides. It remains an important goal to learn to recognize and diagnose these early stages. Tracking the evolution of T cell clones with high throughput sequencing may allow us to better understand these precursor lesions. While there is no evidence that early intervention will prevent the development of extensive ulceration with high morbidity, identification of these early stages may alert us to patients that should be closely followed avoiding immunosuppressive therapies, which could accelerate the course, as seen in some of our patients. Finally, our series highlights the failure of most standard cutaneous T-cell lymphoma therapies in treating this condition. In our experience, allogeneic hematopoietic stem cell transplantation appears to be the only therapeutic option associated with durable partial or complete remission raising the question of whether this treatment should be considered early on and prior to the development of extensive epidermal necrosis and ulceration which is often associated with a poor outcome. However the scarcity of reports of primary cutaneous aggressive epidermotropic cytotoxic T-cell lymphoma still precludes us from establishing therapeutic guidelines.
Supplementary Material
Table 3.
Cytomorphology and immunophenotype of skin biopsies.
| Cases | Cell size | CD2 | CD3 | CD4 | CD5 | CD7 | CD8 | CD30 | CD45RA | CD56 | BetaF1 | TIA-1 | Granz B | EBV | GM1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | m/l | + | + | −/+ | +/− dim | + | + | − | + | − | + | + | + | − | n/a |
| 2 | s/m/l | − | + | − | − | − | + | − | + | − | + | + | −/+ | − | n/a |
| 3 | s/m | n/a | + | − | − | + | + | − | + | + | − | + | n/a | − | − |
| 4 | m/l | − | + | − | + | + | + | − | + | − | + | + | + | − | − |
| 5 | m/l | − | partial+ | − | − | + | + | + in LC cells | n/a | − | + | + | + | − | − |
| 6 | s/m | − | + | − | + | − | + | − | n/a | − | + | + | n/a | − | n/a |
| 7 | s/m | − | + | − | + | + | − | − | + | − | + | + | + | − | − |
| 8 | s/m | − | + | − | − | − | + | weak 30% | + weak | − | − | + | n/a | − | − |
| 9 | m/l | − | + | − | − | + | + | − | n/a | − | n/a | + | + | − | n/a |
| 10 | s/m/l | n/a | + | n/a | rare | n/a | + | n/a | − | 30% | + | n/a | n/a | − | − |
| 11 | m | n/a | + | − | n/a | n/a | + | − | n/a | − | + | n/a | + | n/a | n/a |
| 12 | m/l | n/a | + | − | − | n/a | + | n/a | n/a | n/a | + | + | + | − | n/a |
| 13 | s/m | − | + | − | − | + | + | − | − | − | + | + | − | − | − |
| 14 | m/l | − | + | − | − | + | + | − | subset | − | + | + | − | − | − |
| 15 | m/l | −/+ | + | − | + | + | + | − | + | − | + | − | − | − | − |
| 16 | m | + | + | − | + | + | + | − | − | − | + | + | subset | n/a | − |
| 17 | m | − | + | − | − | − | − | − | − | − | + | subset | + | n/a | − |
| 18 | s/m | + | + | − | − | − | − | − | + | − | − | + | + | − | − |
| 19 | s/m | n/a | + | − | − | + | + | − | − | − | − | + | + | − | − |
| 20 | m | − | + | − | − | − | + | − | + | − | + | + | + | − | − |
| 21 | s/m | + | + | − | − | − | + | − | − | − | − | + | − | − | − |
| 22 | m/l | n/a | + | − | − | + | + | − | n/a | − | + | + | + | − | + |
| 234 | m/l | n/a | + | − | − | + | + | − | + | − | + | + | − | − | − |
| 24 | l | n/a | + | − | − | + | + | 50% exp | subset | + | + | + | + | − | n/a |
| 25 | s/m | n/a | + | − | − | + | + | − | + | − | + | + | − | − | − |
| 26 | s | n/a | + | − | + | + | + | − | n/a | − | + | + | + | − | n/a |
| 27 | s/m | − | + | − | + | + | + | − | + | − | + | + | + | − | + |
| 28 | s | + | + | + (intraep) | + | − | + (intraep) | − | − | − | + | + (intraep), subset | + (intraep), subset | − | − |
| 29 | m | n/a | + | − | − | + | − | n/a | + | − | + | + (golgi) | + | n/a | − |
| 30 | s/m | + | + | − | + | + | − | − | − | − | − | + | + | − | − |
| 31 | m/l | − | + | − | − | + | weak | − | n/a | − | + | + | + | − | n/a |
| 32 | s/m/l | + | + | − | + | + | − | 30% | − | − | + | + | − | − | − |
| 33 | s/m | + | + | − | − | + | weak | − | + | + | weak | + | + | − | − |
| 34 | s/m | + | + | − | − | + | − | n/a | − | − | + | ± | n/a | n/a | − |
| % (+) | 41% of LC | 43 | 100 | 6 | 33 | 74 | 79 | 13 | 61 | 12 | 82 | 97 | 76 | 100 | 12 |
EBV: Epstein-Barr virus; Granz B: granzyme B; intraep: intraepidermal; l: large; LC: large; m: medium; n/a: not applicable; s: small; TIA-1: T-cell intracellular antigen 1.
Acknowledgments
FUNDING
This research was funded in part through the NIH/NCI Cancer Center Support (Grant P30 CA008748), TCF Foundation, Plymouth MN, Therakos.
Footnotes
DISCLOSURES
None
References
- 1.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agnarsson BA, Vonderheid EC, Kadin ME. Cutaneous T cell lymphoma with suppressor/cytotoxic (CD8) phenotype: identification of rapidly progressive and chronic subtypes. J Am Acad Dermatol. 1990;22:569–577. doi: 10.1016/0190-9622(90)70074-r. [DOI] [PubMed] [Google Scholar]
- 3.Berti E, Tomasini D, Vermeer MH, et al. Primary cutaneous CD8-positive epidermotropic cytotoxic T cell lymphomas. A distinct clinicopathological entity with an aggressive clinical behavior. Am J Pathol. 1999;155:483–492. doi: 10.1016/S0002-9440(10)65144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gormley RH, Hess SD, Anand D, et al. Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma. J Am Acad Dermatol. 2010;62:300–307. doi: 10.1016/j.jaad.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 5.Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110:479–484. doi: 10.1182/blood-2006-10-054601. [DOI] [PubMed] [Google Scholar]
- 6.Nofal A, Abdel-Mawla MY, Assaf M, et al. Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma: proposed diagnostic criteria and therapeutic evaluation. J Am Acad Dermatol. 2012;67:748–759. doi: 10.1016/j.jaad.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 7.Robson A, Assaf C, Bagot M, et al. Aggressive epidermotropic cutaneous CD8+ lymphoma: a cutaneous lymphoma with distinct clinical and pathological features. Report of an EORTC Cutaneous Lymphoma Task Force Workshop. Histopathology. 2015;67:425–441. doi: 10.1111/his.12371. [DOI] [PubMed] [Google Scholar]
- 8.Paulli M, Berti E. Cutaneous T-cell lymphomas (including rare subtypes). Current concepts. II. Haematologica. 2004;89:1372–1388. [PubMed] [Google Scholar]
- 9.Wang Y, Li T, Tu P, et al. Primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma clinically simulating pyoderma gangrenosum. Clin Exp Dermatol. 2009;34:e261–262. doi: 10.1111/j.1365-2230.2008.03172.x. [DOI] [PubMed] [Google Scholar]
- 10.Guitart J, Weisenburger DD, Subtil A, et al. Cutaneous gammadelta T-cell lymphomas: a spectrum of presentations with overlap with other cytotoxic lymphomas. Am J Surg Pathol. 2012;36:1656–1665. doi: 10.1097/PAS.0b013e31826a5038. [DOI] [PubMed] [Google Scholar]
- 11.Weenig RH, Comfere NI, Gibson LE, et al. Fatal cytotoxic cutaneous lymphoma presenting as ulcerative psoriasis. Arch Dermatol. 2009;145:801–808. doi: 10.1001/archdermatol.2009.119. [DOI] [PubMed] [Google Scholar]
- 12.Fierro MT, Novelli M, Savoia P, et al. CD45RA+ immunophenotype in mycosis fungoides: clinical, histological and immunophenotypical features in 22 patients. J Cutan Pathol. 2001;28:356–362. doi: 10.1034/j.1600-0560.2001.280704.x. [DOI] [PubMed] [Google Scholar]
- 13.Pongpruttipan T, Sukpanichnant S, Assanasen T, et al. Extranodal NK/T-cell lymphoma, nasal type, includes cases of natural killer cell and alphabeta, gammadelta, and alphabeta/gammadelta T-cell origin: a comprehensive clinicopathologic and phenotypic study. Am J Surg Pathol. 2012;36:481–499. doi: 10.1097/PAS.0b013e31824433d8. [DOI] [PubMed] [Google Scholar]
- 14.Roullet M, Gheith SM, Mauger J, et al. Percentage of {gamma}{delta} T cells in panniculitis by paraffin immunohistochemical analysis. Am J Clin Pathol. 2009;131:820–826. doi: 10.1309/AJCPMG37MXKYPUBE. [DOI] [PubMed] [Google Scholar]
- 15.Merrill ED, Agbay R, Miranda RN, et al. Primary Cutaneous T-Cell Lymphomas Showing Gamma-Delta (gammadelta) Phenotype and Predominantly Epidermotropic Pattern are Clinicopathologically Distinct From Classic Primary Cutaneous gammadelta T-Cell Lymphomas. Am J Surg Pathol. 2016 doi: 10.1097/PAS.0000000000000768. [DOI] [PubMed] [Google Scholar]
- 16.Karkouche R, Ingen-Housz-Oro S, Le Gouvello S, et al. Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma with KIR3DL2 and NKp46 expression in a human immunodeficiency virus carrier. J Cutan Pathol. 2015;42:199–205. doi: 10.1111/cup.12448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.