Abstract
A 47-year-old Caucasian man developed mild diarrhoea associated with more than 10 kg weight loss, severe fatigue and anaemia. Endoscopy demonstrated deposits of AA amyloid within the gastrointestinal tract. He had heavy proteinuria with a serum albumin of 15 g/L consistent with systemic AA amyloidosis. He had no symptoms to suggest an underlying chronic inflammatory condition but had CRP 130 mg/L and SAA 474 mg/L. In an attempt to identify the source of his inflammatory response, he underwent a contrast-enhanced whole-body computed tomography scan, which revealed a necrotising mass lesion in the right kidney consistent with a renal cell carcinoma. It also showed non-mechanical obstruction of the small bowel and, immediately post-imaging, the patient developed intractable vomiting followed by oliguric renal failure requiring haemodialysis. Despite his renal and gut failure, he underwent right radical nephrectomy without further complications. Histology showed complete resection of a clear cell renal cell carcinoma and renal amyloid deposits. Post-surgery, his acute-phase response decreased to normal, consistent with the renal cell carcinoma acting as the inflammatory stimulus. Although he remains dialysis dependent, his gut function improved and he has regained both normal weight and serum albumin. Our case demonstrates partial resolution of AA amyloidosis with removal of the inflammatory source.
Keywords: Nephrotic syndrome, Renal cell carcinoma, AA amyloidosis
Introduction
AA amyloidosis is the most serious potential complication of any chronic inflammatory condition. In the developed world, the most common underlying causes are inflammatory arthritis, particularly rheumatoid arthritis, chronic infections such as bronchiectasis and chronic sepsis complicating paraplegia or drug abuse and inflammatory bowel disease. It has also rarely been described in association with solid organ malignancy, metastatic disease and Hodgkin’s lymphoma [1–8].
Although there are new exciting developments in the treatment for AA amyloidosis, the most consistent feature that has been demonstrated for better outcome is to recognise the underlying source of the inflammatory process and remove it. Our case, once again, demonstrates a favourable outcome after resection of renal cell carcinoma that was driving the systemic amyloidosis.
Case report
A previously entirely healthy 47-year-old Caucasian man developed mild diarrhoea containing mucus and blood associated with more than 10 kg weight loss, severe fatigue and anaemia over a period of 3 months. An endoscopy suggested chronic inflammation macroscopically but biopsies demonstrated deposits of AA amyloid within the stomach, duodenum and the colon (Figs. 1 and 2). Two months later, he presented with nephrotic range proteinuria (4 g/L) with a serum albumin of 15 g/L and eGFR of 108 ml/min, consistent with systemic AA amyloidosis. I123-labelled serum amyloid protein (SAP) scan showed amyloid deposits in the liver, spleen, gut and kidneys. There was no evidence of cardiac amyloidosis on echocardiography.
Fig. 1.
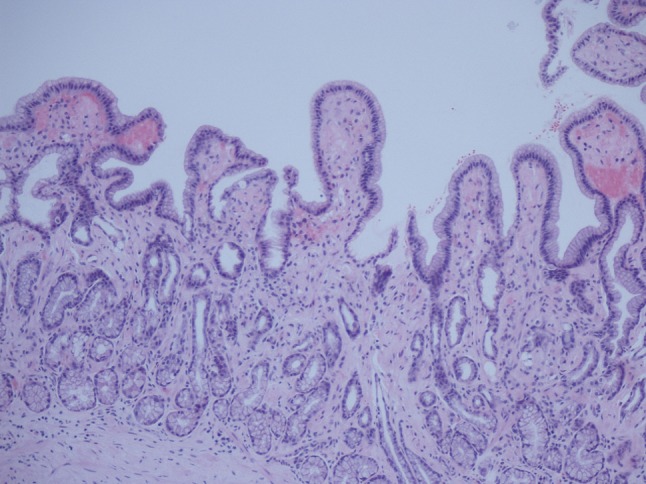
H&E stain of gastric mucosa showing amyloid deposits
Fig. 2.
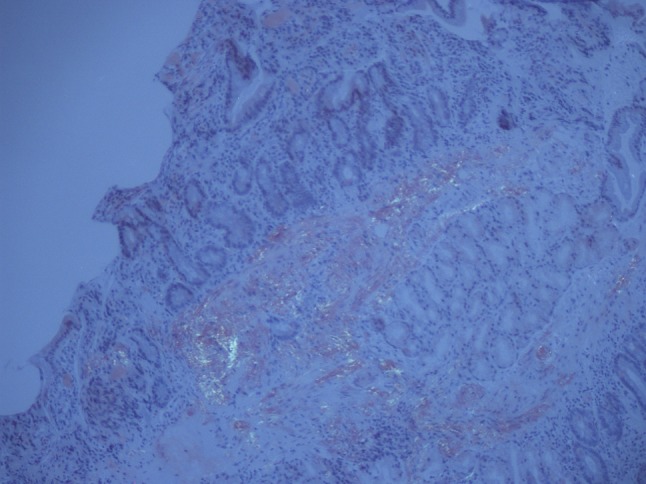
Polarised image of gastric mucosa showing amyloid deposits
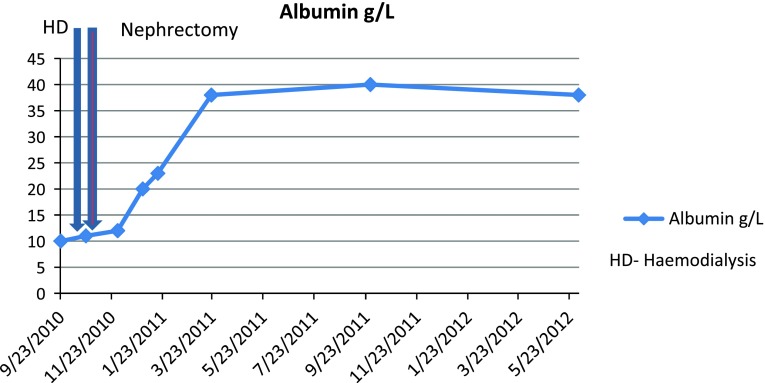
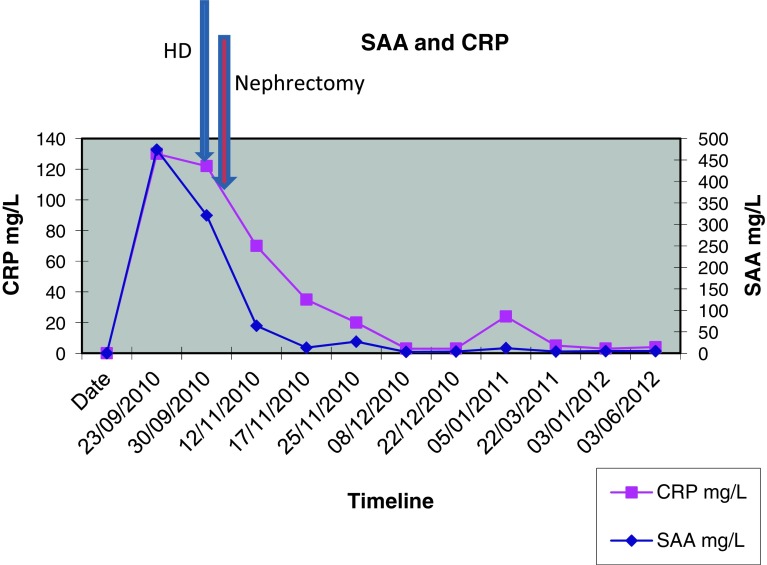
He had no symptoms to suggest an underlying chronic inflammatory condition but had clear biochemical evidence of an upregulated acute-phase response with CRP 130 mg/L and SAA 474 mg/L (median level in healthy blood donors 3 mg/L). An autoantibody profile was normal and, in an attempt to identify the source of his inflammatory response, he underwent a contrast-enhanced whole-body computed tomography (CT) scan. This revealed a necrotising mass lesion in the right kidney consistent with a renal cell carcinoma without any evidence of metastases (Fig. 3). It also showed non-mechanical obstruction of the small bowel and immediately post-imaging. The patient developed intractable vomiting followed by oliguric renal failure requiring haemodialysis. The exact cause of acute kidney injury is unclear but can possibly be down to contrast with CT, pre-renal element with a background of amyloidosis and renal cell carcinoma. Given his pre-existing poor nutritional status and high metabolic requirements, he was started on total parenteral nutrition. Despite his renal and gut failure, he underwent right radical nephrectomy without further complications. Histology showed complete resection of a clear cell renal cell carcinoma and renal amyloid deposits, and it was of AA type (Figs. 4 and 5). Post-surgery, his acute-phase response fell to the normal level, consistent with the renal cell carcinoma acting as the inflammatory stimulus. He remains dialysis dependent, although his gut function improved, and within 2 months, he no longer required nutritional support and had regained both normal weight and serum albumin of 38 g/L (Fig. 6).
Fig. 3.
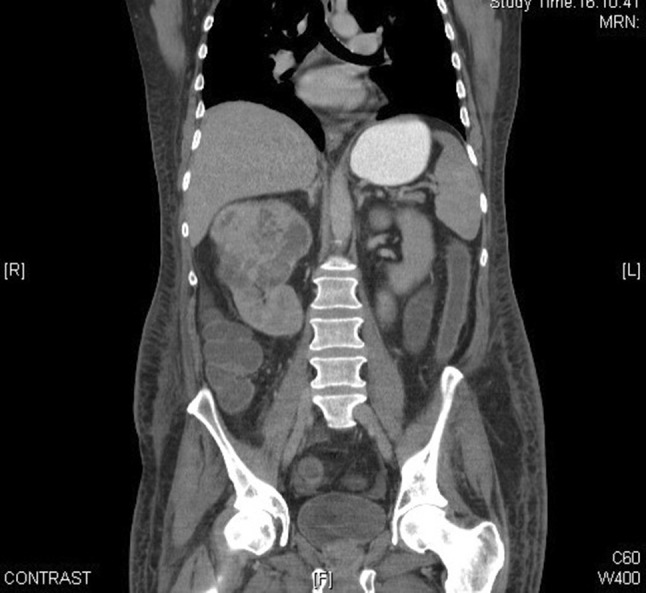
Coronal view of contrast-enhanced computed tomography (CT) demonstrating renal cell carcinoma
Fig. 4.
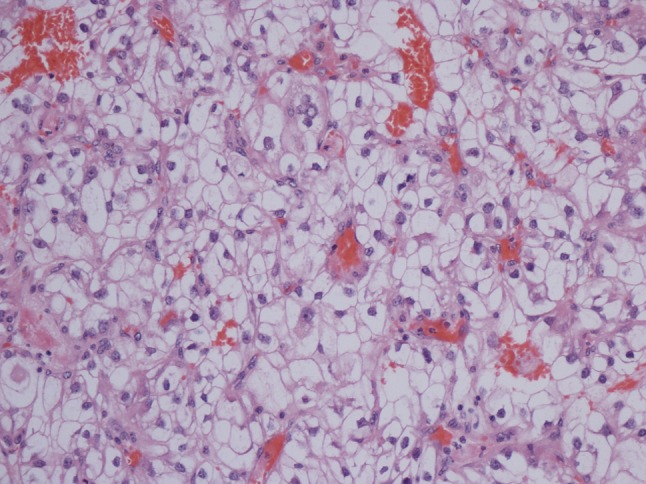
H&E stain of kidney tissue demonstrating clear cell carcinoma
Fig. 5.
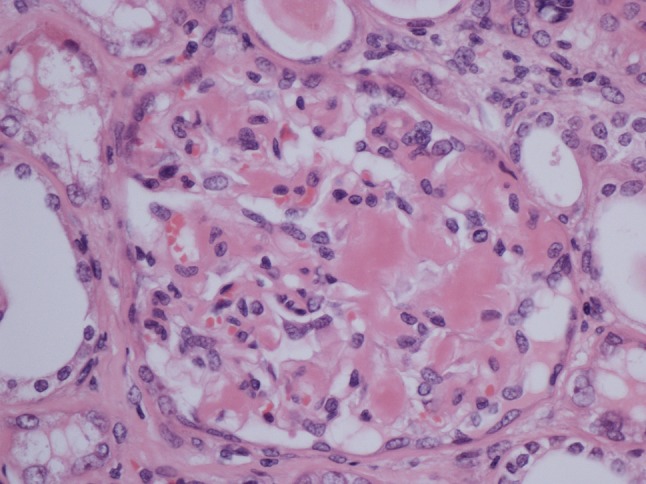
H&E stain of the glomerulus showing hyaline deposits in the glomerular mesangium, demonstrating amyloid protein
Fig. 6.
Timeline and albumin
Discussion
AA (systemic) amyloidosis involves the deposition of amyloid protein in tissues. The amyloid fibrils are derived from N-terminal cleavage fragments of the hepatic acute-phase reactant serum amyloid A protein (SAA). SAA is an apolipoprotein constituent of high-density lipoprotein which is synthesised by hepatocytes under the transcriptional regulation of the proinflammatory cytokines IL-6, IL-1 and TNF-alpha. Like CRP, SAA is a highly dynamic acute-phase response protein and circulating levels can increase from a baseline of <3 mg/L to as high as 2000 mg/L. High levels are maintained throughout an inflammatory response but, after termination of the stimulus levels, fall with a half life of <24 h [9, 10]. Sustained high circulating levels of SAA are a prerequisite for the development of AA amyloidosis but are clearly not sufficient, as amyloidosis develops in <5 % of the at-risk population. The nature and role of other risk factors is not entirely clear, although SAA isotype, extracellular matrix components and macrophage factors have all been implicated.
AA amyloidosis is a multisystem disease and amyloid deposits can be found in almost all sites on biopsy. Patients almost always present with proteinuric renal dysfunction. More extensive disease can produce hepatosplenomegaly and gut dysfunction, usually causing diarrhoea and weight loss. Symptomatic cardiac or nervous system amyloidosis occurs in <2 % of cases and is generally evidence of very advanced disease.
Malignancy is the underlying inflammatory driver in <7 % of AA amyloidosis. The association of amyloidosis and renal cell carcinoma was first reported by Ask-Upmark in 1940, who described 18 cases. Large autopsy series suggest that renal cell carcinoma is very much the most common solid organ malignancy to cause AA amyloidosis, accounting for 25–42 % of reported cases, but that, conversely, amyloid deposits are only seen in 2.1–3.2 % of patients who died with a renal cell carcinoma [8, 11]. Renal cell tumours are known to produce IL-6, which can act as an autocrine growth factor. High levels of tumour-derived IL-6 can result in increased circulating SAA and CRP [12]. Indeed, these acute-phase proteins have been used as tumour markers and anti-IL-6 antibodies are now undergoing phase 2/3 trials in metastatic renal cell carcinoma [13].
There have been 20 cases of the co-existence of amyloidosis and renal cell carcinoma reported in the literature. In two cases, the amyloidosis appears to have been of type AL, complicating concomitant myeloma and, in the other cases, the amyloid was of type AA. Amyloid deposits was found to occur in various tissues at extracellular sites in the kidney, liver, spleen, adrenals and mucosa of the upper respiratory tract. Four cases of retroperitoneal amyloid deposits have also been reported. AA amyloidosis has been induced by other solid organ malignancies, gastrointestinal stromal tumours and bladder cancer. Benign neoplasms like hepatic adenoma and hyaline vascular Castleman’s disease are also associated with AA amyloidosis (Table 1).
Table 1.
Review of case reports and case series published in the literature
| Case | Age (years) | Sex | Features | Diagnosis | Treatment | Outcome |
|---|---|---|---|---|---|---|
| 1 | 54 | Female | Nephrotic syndrome, AA amyloidosis in the kidney | Rt kidney clear cell carcinoma and AA amyloidosis | Nephrectomy | Died secondary to cerebral metastases after 2 years |
| 2 | 61 | Male | Haematuria | Left kidney RCC, retroperitoneal amyloidosis | Nephrectomy and excision of retroperitoneal excision | Well up to reported date |
| 3 | 58 | Male | Haematuria and renal failure | Familial Mediterranean fever, urothelial carcinoma, papillary adenoma and AA amyloidosis | Right radical nephrectomy | Survived up until the case was reported |
| 4 | 72 | Male | Haematuria, nephrotic syndrome, renal insufficiency | Papillary carcinoma of the bladder, gastric adenoca and AA amyloidosis of the kidney | For conservative management | Died within a year of diagnosis |
| 5 | 72 | Female | Fleshy growth of conjunctiva, hydronephrosis | AA amyloidosis of conjunctiva and para nasal sinuses, papillary carcinoma of the left kidney | Left nephrectomy | Well |
| 6 | 58 | Female | Sub-nephrotic range proteinuria, renal impairment | Clear cell carcinoma in the inferior pole of the left kidney; AA amyloidosis in the left kidney | Left radical nephrectomy | Well with CKD 3 |
| 7 | 69 | Female | Nephrotic syndrome, acute renal failure | Hypernephroma in horseshoe kidney, AA amyloidosis | Right nephrectomy | Died several days post-operatively due to other complications (CVE, af) |
| 8 | 69 | Male | Microcytic anaemia | GIST in stomach; AA amyloidosis in spleen, adrenals, pancreas | Gastrectomy, splenectomy, left adrenalectomy, partial pancreatectomy | Died after 6 months due to liver metastases |
| 9 | 57 | Male | Nephrotic syndrome, haematuria, acute kidney injury | Small cell carcinoma of the bladder. AA amyloidosis in gut and kidneys | Surgery for removal of cancer, local radiotherapy and chemotherapy. | Haemodialysis was started 6 months after initial diagnosis, patient died 13 months from diagnosis. |
| 10 | 62 | Female | Nephrotic syndrome | Renal cell carcinoma and AA amyloid deposits in kidney and gut | Nephrectomy | Resolution of gut amyloid after 30 months |
| 11a | 67 | Male | Slowly progressive polyneuropathy | Left clear cell carcinoma of kidney, Amyloid deposits in Kidney and Intramuscular arteries. | Left nephrectomy | 2 years after diagnosis developed lung and liver metastases |
| 11b | 67 | Male | Paresthesia and haematuria | Hypernephroma of left kidney. AA Amyloid deposits in muscles and nerves. | Nephrectomy | Well |
| 12 | 43 | Male | Nephrotic syndrome and acute kidney injury | Ulcerative colitis and renal cell carcinoma; AA amyloid deposits in the kidney | Nephrectomy | Started haemodialysis 6 months after nephrectomy |
| 13 | 34 | Female | Nephrotic syndrome, obstructive jaundice; chronic contraceptive use | Hepatic adenoma; AA amyloid deposits in the liver and kidney | Partial right hepatectomy | Haemodialysis-dependent post-operatively, renal transplant 10 months post-operatively and is currently well |
| 13 | 20 | Female | Nephrotic syndrome | Castleman’s disease and AA amyloid deposits in the kidney. | Resection of retroperitoneal mass | Developed ESRD on dialysis, had cadaveric renal transplant after 6 years and is well to date |
| 14 | 80 | Male | Sub-nephrotic proteinuria; 3 months later, presented with nephrotic syndrome | Clear cell carcinoma of the left kidney; AL type amyloidosis in the kidneys and GI tract | Left nephrectomy | Well up until the case was reported |
The majority of cases presented with proteinuric renal impairment rather than direct symptoms from the tumour. A retrospective review of 68 cases has identified two main types of amyloid deposit in the kidney: glomerular and vascular. The glomerular form was mainly described as mesangial nodular or mesangiocapillary types and was associated with nephrotic range proteinuria. The vascular form was associated with lesser proteinuria. In the study, it was found that age, tubular atrophy, glomerular deposit pattern and arteriolar amyloid load were predictors of renal insufficiency. Haematuria was found in cases where glomerular amyloid load and subepithelial deposits were important. Either partial or complete remission of amyloidosis was achieved in the cases after removal of the cancer which acted as the main driver [12, 14] (Table 1).
Untreated, AA amyloidosis is a serious disease with a significant morbidity and mortality due to end-stage renal disease [1]. Patients with persistently high circulating levels of SAA are at particular risk of complications, including progression to renal failure, and of death [15, 16] (Table 2). In a review of 374 cases of AA amyloidosis, the mean survival time from diagnosis was 133 months, with older age, reduced serum albumin concentration and end-stage renal failure at baseline, and the degree by which the SAA concentration was elevated during follow-up were poor prognostic factors [1]. Successful treatment of the underlying inflammatory process, including with surgical resection of the tumour, as demonstrated in our case, can lead to the stabilisation of or improvement in renal function, reduction in protein excretion and partial resolution of amyloid deposits [17–23] (Fig. 7). In our patient case, the renal dysfunction appears irreversible, but we expect to see amyloid regression from the liver and spleen in time and, provided there is no evidence of tumour recurrence, the long-term management plan is renal transplantation.
Table 2.
Case report and case series reported
| No. | Title | Authors | Journal | PMID |
|---|---|---|---|---|
| 1 | Remission of nephrotic syndrome in amyloidosis associated with a hypernephroma | Tang AL, Davies DR, Wing AJ | Clin Nephrol. 1989 Nov; 32(5):225–8 | 2582647 |
| 2 | Diffuse retroperitoneal amyloidosis due to renal cell carcinoma | Coakley FV, Hricak H, Presti JC Jr, Small EJ | Br J Radiol. 1999 Apr; 72(856):412–3 | 10474508 |
| 3 | Concomitant amyloidosis, renal papillary carcinoma and ipsilateral pelvicalyceal urothelial carcinoma in a patient with familial Mediterranean fever | Kirkpantur A, Baydar DE, Altun B, Karcaaltincaba M, Aki T, Yuksel S, Turgan C | Amyloid. 2009 Mar; 16(1):54–9 | 19291516 |
| 4 | Synchronous carcinomas of stomach and bladder together with AA amyloidosis | Ersoy A, Filiz G, Ersoy C, Kahvecioglu S, Kanat O, Vuruskan H, Gullulu M | Nephrology (Carlton). 2006 Apr; 11(2):120–3 | 16669973 |
| 5 | Secondary amyloidosis of the conjunctiva and mucosa of upper respiratory tract associated with renal cell carcinoma: case report and review of literature | Ibrahim TM, Iheonunekwu N | Niger J Med. 2009 Apr–Jun; 18(2):172–4. Review | 19630323 |
| 6 | Renal cell carcinoma and systemic amyloidosis: demonstration of AA protein and review of the literature | Vanatta PR, Silva FG, Taylor WE, Costa JC | Hum Pathol. 1983 Mar; 14(3):195–201 | 6832767 |
| 7 | AA amyloidosis due to renal cell carcinoma in a horseshoe kidney | García-Agudo R, Moyano MJ, Aoufi S, Milán JA | Nefrologia. 2008; 28(1):109–10. Spanish | 18336144 |
| 8 | Secondary amyloidosis and gastrointestinal stromal tumors. A case report and discussion of pathogenesis | Overstreet K, Barone RM, Robin HS | Arch Pathol Lab Med. 2003 Apr; 127(4):470–3 | 12683877 |
| 9 | Systemic AA amyloidosis and nephrotic syndrome associated with small cell carcinoma of the bladder | Kanat O, Evrensel T, Filiz G, Usta M, Baskan E, Dilek K, Manavoglu O | Nephrol Dial Transplant. 2003 Nov; 18(11):2453–4 | 14551390 |
| 10 | Clinical and histological resolution of systemic amyloidosis after renal cell carcinoma removal | Karsenty G, Ulmann A, Droz D, Carnot F, Grünfeld JP | Nephron. 1985; 40(2):232–4 | 4000353 |
| 11 | Nature of amyloid deposits in hypernephroma. Immunocytochemical studies in 2 cases associated with amyloid polyneuropathy | Dalakas MC, Fujihara S, Askanas V, Engel WK, Glenner GG | Am J Pathol. 1984 Sep; 116(3):447–54 | 6383062 |
| 12 | The possible pathogenesis of AA type amyloidosis in a patient with ulcerative colitis and renal cell carcinoma | Kostadinova-Kunovska S, Petrusevska G, Grcevska L, Banev S, Dzikova S, Bogoeva B, Polenaković M | Acta Med Croatica. 2006 Jun; 60(3):251–4. Croatian | 16933838 |
| 13 | Systemic AA amyloidosis induced by benign neoplasms | Bestard Matamoros O, Poveda Monje R, Ibernon Vilaró M, Carrera Plans M, Grinyó Boira JM | Nefrologia. 2008; 28(1):93–8. Spanish | 18336138 |
| 14 | Case of nephrotic syndrome due to AL-type primary amyloidosis associated with renal cell carcinoma | Takano M, Suda N, Ichihara A, Konishi K, Sasamura H, Hayashi K, Hashiguchi A, Miyajima A, Murai M, Itoh H | Nihon Jinzo Gakkai Shi. 2007; 49(8):1014–9. Japanese | 18186230 |
Fig. 7.
SAA and CRP graph
Conclusion
AA amyloidosis is always a complication of an underlying chronic inflammatory state [24]. In 6 % of cases, the nature of the underlying condition is not overt at presentation [1]. In these cases, a comprehensive search for the underlying condition must be made, as effective treatment which terminates the acute-phase response will prevent further amyloid deposition and, in some cases, may permit sufficient amyloid regression to preserve renal function. Renal cell carcinoma is a rare but potentially fully resectable cause of AA amyloidosis, which is often completely asymptomatic and should be actively sought in patients of AA amyloidosis of unknown aetiology.
Acknowledgments
Conflict of interest
We do not declare any conflicts of interest.
Contributor Information
Adarsh Babu, Phone: +44-117-9505050, Phone: +44-7871-579576, Email: Adarsh.Babu@nbt.nhs.uk, Email: Adarshbabu81@yahoo.com.
Helen Lachmann, Phone: +44-20-76792000, Email: h.lachmann@medsch.ucl.ac.uk.
Tom Pickett, Phone: +44-845-4226762, Email: Tom.Pickett@glos.nhs.uk.
Preetham Boddana, Phone: +44-845-4228371, Email: Preetham.Boddana@glos.nhs.uk.
Linmarie Ludeman, Phone: +44-845-4222222, Email: Linmarie.Ludeman@glos.nhs.uk.
References
- 1.Lachmann HJ, Goodman HJ, Gilbertson JA, et al. Natural history and outcome in systemic AA amyloidosis. N Engl J Med. 2007;356:2361–2371. doi: 10.1056/NEJMoa070265. [DOI] [PubMed] [Google Scholar]
- 2.Pras M, Franklin EC, Shibolet S, Frangione B. Amyloidosis associated with renal cell carcinoma of the AA type. Am J Med. 1982;73:426–428. doi: 10.1016/0002-9343(82)90747-1. [DOI] [PubMed] [Google Scholar]
- 3.Jaakkola H, Törnroth T, Groop PH, Honkanen E. Renal failure and nephrotic syndrome associated with gastrointestinal stromal tumour (GIST)—a rare cause of AA amyloidosis. Nephrol Dial Transplant. 2001;16:1517–1518. doi: 10.1093/ndt/16.7.1517. [DOI] [PubMed] [Google Scholar]
- 4.Agha I, Mahoney R, Beardslee M, Liapis H, Cowart RG, Juknevicius I. Systemic amyloidosis associated with pleomorphic sarcoma of the spleen and remission of nephrotic syndrome after removal of the tumor. Am J Kidney Dis. 2002;40:411–415. doi: 10.1053/ajkd.2002.34545. [DOI] [PubMed] [Google Scholar]
- 5.Mata M, Lohr T, Lakshminarayanan M, Lo KH, Prabhakar U, Centocor, Inc., Malvern, PA Serum amyloid A (SAA), C-reactive protein (CRP) and IL-6 as prognostic indicators in renal cell carcinoma and prostate cancer. J Clin Oncol. 2004;22(14S):9711. [Google Scholar]
- 6.Wood SL, Rogers M, Cairns DA, Paul A, Thompson D, Vasudev NS, et al. Association of serum amyloid A protein and peptide fragments with prognosis in renal cancer. Br J Cancer. 2010;103(1):101–111. doi: 10.1038/sj.bjc.6605720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger L, Sinkoff MW. Systemic manifestations of hypernephroma review of 273 cases. Am J Med. 1957;22:791–796. doi: 10.1016/0002-9343(57)90129-8. [DOI] [PubMed] [Google Scholar]
- 8.Dictor M, Hasserius R. Systemic amyloidosis and non-hematologic malignancy in a large autopsy series. Acta Pathol Microbiol Scand A. 1981;89(6):411–416. doi: 10.1111/j.1699-0463.1981.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 9.Lavie G, Zucker-Franklin D, Franklin EC. Degradation of serum amyloid A protein by surface-associated enzymes of human blood monocytes. J Exp Med. 1978;148:1020–1031. doi: 10.1084/jem.148.4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westermark GT, Sletten K, Grubb A, Westermark P. AA-amyloidosis. Tissue component-specific association of various protein AA subspecies and evidence of a fourth SAA gene product. Am J Pathol. 1990;137(2):377–383. [PMC free article] [PubMed] [Google Scholar]
- 11.Azzopardi JG, Lehner T. Systemic amyloidosis and malignant disease. J Clin Pathol. 1966;19(6):539–548. doi: 10.1136/jcp.19.6.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miki S, Iwano M, Miki Y, et al. Interleukin-6 (IL-6) functions as an in vitro autocrine growth factor in renal cell carcinomas. FEBS Lett. 1989;250:607–610. doi: 10.1016/0014-5793(89)80805-1. [DOI] [PubMed] [Google Scholar]
- 13.Rossi J-F, Négrier S, James ND, et al. A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer. Br J Cancer. 2010;103:1154–1162. doi: 10.1038/sj.bjc.6605872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gertz MA, Kyle RA. Secondary systemic amyloidosis: response and survival in 64 patients. Medicine (Baltimore) 1991;70:246–256. doi: 10.1097/00005792-199107000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka F, Migita K, Honda S, et al. Clinical outcome and survival of secondary (AA) amyloidosis. Clin Exp Rheumatol. 2003;21:343–346. [PubMed] [Google Scholar]
- 16.Falck HM, Maury CP, Teppo AM, Wegelius O. Correlation of persistently high serum amyloid A protein and C-reactive protein concentrations with rapid progression of secondary amyloidosis. Br Med J (Clin Res Ed) 1983;286:1391–1393. doi: 10.1136/bmj.286.6375.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovat LB, Madhoo S, Pepys MB, Hawkins PN. Long-term survival in systemic amyloid A amyloidosis complicating Crohn’s disease. Gastroenterology. 1997;112:1362–1365. doi: 10.1016/S0016-5085(97)70150-1. [DOI] [PubMed] [Google Scholar]
- 18.Waldenström H. On the formation and disappearance of amyloid in man. Acta Chir Scand. 1928;63:479–530. [Google Scholar]
- 19.Shimojima Y, Takei Y, Tazawa K, et al. Histopathological regression of systemic AA amyloidosis after surgical treatment of a localized Castleman’s disease. Amyloid. 2006;13:184–186. doi: 10.1080/13506120600876930. [DOI] [PubMed] [Google Scholar]
- 20.Ortiz-Santamaría V, Olivé A, Valls-Roc M, Tena X. Treatment of AA amyloid with chlorambucil. Rheumatology (Oxford) 2002;41:833. doi: 10.1093/rheumatology/41.7.833. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura T, Yamamura Y, Tomoda K, Tsukano M, Shono M, Baba S. Efficacy of cyclophosphamide combined with prednisolone in patients with AA amyloidosis secondary to rheumatoid arthritis. Clin Rheumatol. 2003;22:371–375. doi: 10.1007/s10067-003-0763-9. [DOI] [PubMed] [Google Scholar]
- 22.Mpofu S, Teh LS, Smith PJ, Moots RJ, Hawkins PN. Cytostatic therapy for AA amyloidosis complicating psoriatic spondyloarthropathy. Rheumatology (Oxford) 2003;42:362–366. doi: 10.1093/rheumatology/keg101. [DOI] [PubMed] [Google Scholar]
- 23.Berglund K, Thysell H, Keller C. Results, principles and pitfalls in the management of renal AA-amyloidosis; a 10–21 year followup of 16 patients with rheumatic disease treated with alkylating cytostatics. J Rheumatol. 1993;20:2051–2057. [PubMed] [Google Scholar]
- 24.Uda H, Yokota A, Kobayashi K, et al. Two distinct clinical courses of renal involvement in rheumatoid patients with AA amyloidosis. J Rheumatol. 2006;33(8):1482–1487. [PubMed] [Google Scholar]