Figure 2.
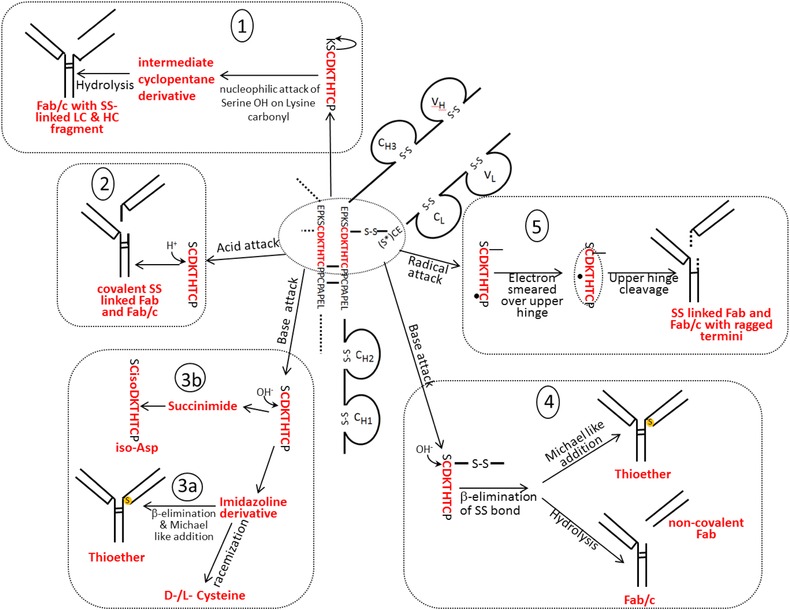
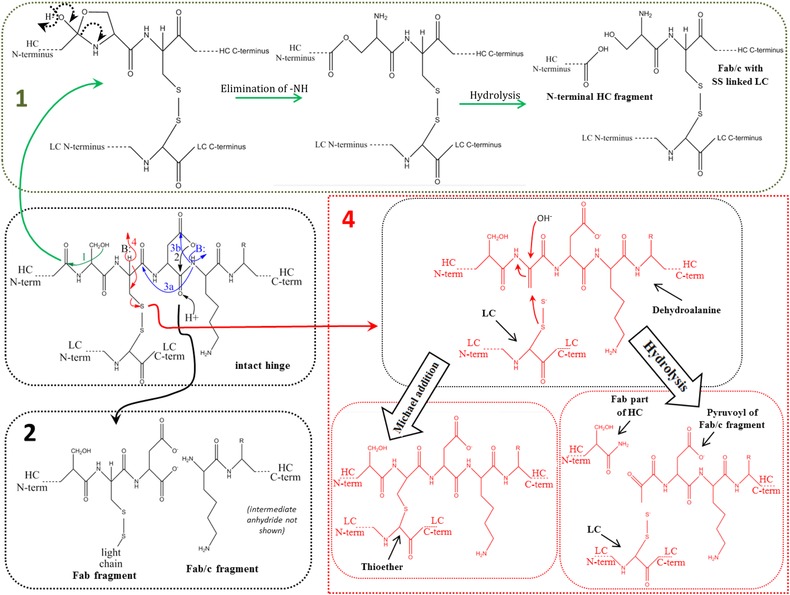
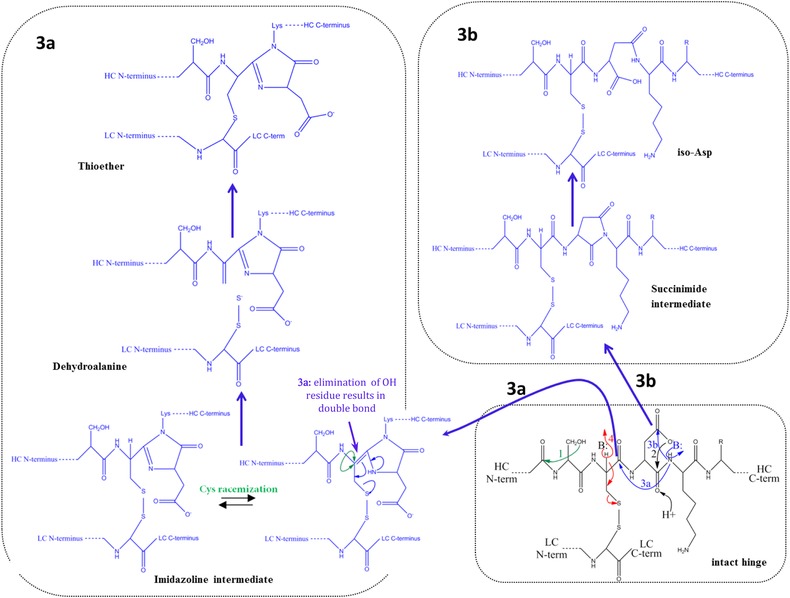
Main fragmentation and modification processes in the upper hinge region. Mechanism 1 (A and B): Nucleophilic attack of serine OH on lysine carboxyl at pH 3–8 with subsequent hydrolysis (according to Vlasak et al. 11). Mechanism 2 (A and B): C‐terminal Asp cleavage is induced by protonation of the carbonyl of the peptide bond between Asp and Lys. Ring closure between the carbonyl carbon and the oxygen of the Asp carboxylgroup is succeeded by hydrolysis of the peptide bond (according to Vlasak et al. 11). This reaction is preferred under acidic conditions (below pH 5). Mechanism 3a (A and C): Abstraction of a proton from the nitrogen of the peptide bond between the upper hinge Asp and Lys and ring closure with the carbonyl of the Asp‐Cys peptide bond leads to an imidazoline derivative whose racemization leads to d‐Cys. Further β‐elimination of the disulfide bond and subsequent Michael addition leads to a thioether linkage. Mechanism 3b: Abstraction of a proton from the nitrogen of the peptide bond between the upper hinge Asp and Lys and ring closure with the carbonyl of the Asp side chain leads to a succinimide intermediate and subsequent hydrolysis to iso‐aspartate (according to Amano et al. 21). Mechanism 4 (A and B): Base attack leads to β‐elimination of the disulfide bond and formation of dehydroalanine. Subsequent hydrolysis leads to a noncovalent Fab amide and an Fab/c (pyruvoyl at the N‐terminus of the cleaved HC), whereas Michael‐like addition leads to Thioether formation (according to Vlasak et al. and Cohen et al. 11, 23). This reaction is preferred under basic conditions. Mechanism 5 (A): Radical attack of the C‐terminal upper hinge disulfide bond leads to sulfenic acid (Cys‐SOH) on one Cys and a thiyl radical (Cys‐S●) on the other 18. The thiyl radical transfers its electron to the upper hinge and induces backbone cleavage somewhere within the upper hinge. This forms Fab and Fab/c fragments with ragged termini 18, 23. The upper hinge histidine is assumed to be an important radical center during this process 19.