Abstract
Objectives
The development of novel agents and an ageing population has led to an increasing number of patients with follicular lymphoma (FL) living longer with their disease. Health‐related quality of life (HRQOL) is a priority for patients and should guide clinical decisions. The Myeloma Patient Outcome Scale (MyPOS), originally developed for myeloma, was validated in a cross‐sectional survey recruiting 124 FL patients.
Methods
Content and construct validity, structural validity using confirmatory factor analyses, reliability and acceptability were evaluated.
Results
Three subscales were indicated: symptoms and function, emotional response, and healthcare support. MyPOS symptom and function scores were higher (worse) in participants with poorer ECOG performance status (F=26.2, P<.000) and discriminated between patients on and off treatment. Good convergent and discriminant validity in comparison to the EORTC‐QLQ‐C30 and FACT‐Lym were demonstrated. Internal consistency was good; α coefficient 0.70‐0.95 for the total MyPOS score and subscales.
Conclusion
The MyPOS is valid, reliable and acceptable, and can be used to support clinical care of FL patients. This is the first measurement tool developed specially for use in clinical practice that has been validated for use in people with FL. Further longitudinal validation is now required to support its use in outcome measurement.
Keywords: factor analysis, Follicular lymphoma, non‐Hodgkin lymphoma, psychometrics, quality of life, questionnaire validation
1. Introduction
In Europe 93 500 new cases of non‐Hodgkin's lymphoma (NHL) were diagnosed in 2012, making it the 11th most common cancer. Today 250 000 people are living with NHL in Europe up to 5 years after being diagnosed.1 Follicular lymphoma (FL) is the most common indolent form of NHL, accounting for 20% of all NHL in the USA and Western Europe.2, 3 FL is more common in women, median age at diagnosis is 65 years old and incidence increases with age.2 As populations in Western Europe and the United States increasingly age, and advances in treatment continue to improve survival, healthcare systems face growing numbers of people with FL needing long‐term care.
FL remains incurable despite considerable improvements in treatment options over the last four decades. The disease trajectory is characterised by patterns of remission and relapse and is without a defined optimal management strategy.4 Active monitoring for patients with low disease burden is a common strategy but is associated with anxiety as patients anticipate inevitable progression of the disease.5 Moreover, because of the increase in survival, long‐term effects of therapy accumulate, among them toxicity‐related symptoms, persistent fatigue, depression and anxiety.5, 6, 7, 8, 9, 10, 11 These long‐term effects have been shown in a recent systematic review to impact all dimensions of health‐related quality of life (HRQOL) even at more than 5 years post‐diagnosis.10
Despite these long‐term effects, research on HRQOL in NHL and FL patients is lacking. Only four studies so far have specifically focused on FL patients, documenting the effect of different modes of treatment on return to work and emotional well‐being.8, 11, 12, 13 Two systematic reviews of HRQOL in NHL present overall results for this group of haematological cancer patients.10, 14 Both conclude that the paucity of evidence is caused in part by a lack of disease‐specific HRQOL measures. Indeed, prior to 2013 no HRQOL measure was validated for use in NHL. The Functional Assessment of Cancer Therapy—Lymphoma (FACT‐Lym)15 is the only questionnaire available, however, its validation study does not identify the proportion of FL in the sample. A further NHL‐specific module of the European Organisation for Research and the Treatment of Cancer (EORTC) is currently being developed.16 Moreover, as HRQOL and other patient‐reported outcome measures are increasingly used in the clinical care of oncology patients,17, 18, 19 a different type of measure, suitable for routine clinical use, is needed. Measures developed for clinical use differ from HRQOL measures developed for use in research by a special focus on content and face validity, acceptability and interpretability.20
The Myeloma Patient Outcome Scale (MyPOS) is such a measure, designed using extensive patient interviews, it is brief and suitable for use in clinical practice for measuring myeloma‐specific HRQOL.21 Given the comparability of disease trajectories (ranging from relatively indolent forms of FL and myeloma‐spectrum diseases to relapsed/refractory stages) and treatment‐related side effects,11, 13, 22, 23 suitability of MyPOS for use in FL is plausible. The objectives of this study are to test the construct and structural validity, examine the content validity and acceptability, and evaluate the reliability of MyPOS in FL patients.
2. Methods
2.1. Setting and population
Between June and August 2013, patients with FL and myeloma were recruited for a self‐completion, cross‐sectional survey from outpatient clinics and inpatient wards in 13 hospital trusts in England, including a mix of tertiary and district general hospitals (see Acknowledgements for list of collaborators). In this paper, we focus on validation results for the sample of FL patients. Full details about the methods are reported in the validation study with myeloma patients.24
Patients were included if they were 18 years or older, had a confirmed histological diagnosis of FL, were aware of their diagnosis, and could give written informed consent. Patients were excluded if they were too unwell, symptomatic or distressed to take part (as judged by the clinical team), had severe neutropenia, or were unable to understand written or spoken English.
Research Ethics Committee approval was granted by the South East London REC‐3 (ref 10/H0808/133) and local approvals were obtained from each Research & Development department of all participating sites. All participants gave written consent to take part and participation was voluntary. Completed questionnaires were screened for clinically important issues and where necessary the participant's consent was sought to feed such issues back to the clinical team.
2.2. Procedure
Consecutive (all available) patients seen on wards or in outpatient clinics at participating sites were screened by clinical teams for eligibility and if eligible offered the opportunity to participate in the survey by research staff. Participants were given the choice to complete the questionnaire at the point of recruitment or at a later date and to return the survey by post. Demographic and clinical data were extracted from medical records by research nurses at recruitment.
2.3. Study measures
Questionnaire booklets contained MyPOS alongside two further measures for validation purposes; FACT‐LYM and EORTC‐QLQ‐C30. The latter two measures were chosen for testing convergent and divergent construct validity. They represent the only lymphoma‐specific HRQOL questionnaire and the EORTC's core cancer questionnaire, which has been validated in lymphoma.25
The MyPOS is a 30‐item questionnaire, a myeloma‐specific version of the Palliative Care Outcome Scale (http://pos-pal.org/). The questionnaire starts with an open question “What are your main problems or concerns at the moment?”. This is followed by 27 structured items on a 5‐point Likert scale. There is one further open question designed to pick up problems not covered in the structured items “Please list any other symptoms not mentioned above, and tick one box to show how they have affected you over the past week”. The MyPOS was developed through qualitative investigation of the issues most important to the HRQOL of people with myeloma.24 It was initially validated in a sample of 380 patients. Factor analysis confirmed three subscales; (i) symptoms and function (14 items covering physical symptoms and functional impairments); (ii) emotional response (eight items describing the emotional impact of the disease); and (iii) healthcare support (five items on information needs and satisfaction with healthcare).24 The MyPOS is a valid and reliable tool for use in the routine clinical care of myeloma patients.24 For validation, the total MyPOS score (formed by summing the scores of the 27 structured items), and subscale scores (formed by summing the item scores within each subscale) were used for analyses. Higher scores represent worse HRQOL.
The FACT‐LYM includes the Functional Assessment of Cancer Therapy—General (FACT‐G) and fifteen additional disease‐specific items. Overall it contains 42 questions, each on a 5‐point Likert scale, and can be combined into five well‐being subscales: (i) physical (seven items); (ii) social/family (seven items); (iii) emotional (six items); (iv) functional (seven items); and (v) lymphoma‐specific additional concerns (15 items, covering symptoms, patient concerns about symptoms, emotional problems and future care plans). The validity and reliability of the FACT‐LYM was tested in patients with NHL, with preliminary tests confirming its good internal consistency and a full validation study showing its validity and sensitivity to change.26, 27 For convergent and divergent validity testing, only the individual subscales of the FACT‐LYM were used. Higher scores represent better functioning.
The EORTC‐QLQ‐C30 has 30 items on a 4‐point scale, combined into five subscales (i) physical; (ii) role; (iii) cognitive; (iv) emotional; and (v) social functioning. In addition, two 7‐point items form a global health status scale. All subscale scores were transformed to a 0‐100 scale following the recommended method.28 High scores on functional sub‐scales and the global scale represent better functioning, whereas high scores on symptom scales represent a worse symptom burden and poor HRQOL. Its validation status in NHL is not well supported, the only psychometric study having been conducted in a Greek sample of 80 NHL patients.25
Clinical characteristics included date of diagnosis, the stage of disease at diagnosis,29 current treatment phase ((i) active monitoring/observation; (ii) on active treatment; (iii) on maintenance treatment; (iv) progressive/palliative disease), number of lines of treatment (including the current treatment), and Eastern Cooperative Oncology Group performance status (ECOG PS).30
2.4. Statistical analysis
The recruitment target was 111 participants, for detecting differences in MyPOS symptom and function subscale scores according to performance status, in a one‐way ANOVA with small effects (0.03) and 80% power. Data was double‐entered into an SPSS database. All analyses were completed in SPSS v 22.0,31 and in AMOS v. 22.0.32
The following psychometric properties were tested:
Construct validity: structural validity (factor analysis). Confirmatory factor analysis (CFA) was used to test the retention of the three‐factor model (symptom and function, emotional response, and healthcare support) previously established for MyPOS in patients with myeloma.24 The question about sex (item 6 on the MyPOS) was excluded from analyses due to a large proportion of missing data. Item parcelling, using the median score across four items related to gastro‐intestinal symptoms (diarrhoea, constipation, nausea and vomiting) was also used to increase the participant to item ratio, in line with the recommend 5:1 ratio.33, 34 Factorability of the matrix was tested using Bartlett's test of sphericity, seeking a large significant value indicating the MyPOS matrix was significantly different from a matrix with no correlations.35 Given our small sample size, factorability was further confirmed in SPSS using the Kaiser‐Meyer‐Olkin Measure of Sampling Adequacy (MSA), applying Kaiser's criteria for ‘good factor‐analytic’ of MSA≥0.80.36 Initial CFA was carried out on the simplest uni‐dimensional model, loading all items onto one factor. Different three‐factor solutions were tested in contrast to this model. Three goodness‐of‐fit indices were used to compare models: Chi‐square, Comparative Fit Index (CFI), and the Root mean square error of approximation (RMSEA). Because of the known limitation of the Chi‐square χ2 statistic with its sensitivity to sample size, the supplementary index; ratio of χ2 to degrees of freedom (χ2/df) was used. A ratio of <2 is considered to reflect good model fit.37 The CFI ranges from 0 to 1 and compares the model against the null model; a value >0.95 is considered representative of good fit.38 For the RMSEA, 0.08 represents a reasonable fit.39 Factor loadings of items on predicted factors were considered acceptable when above 0.30.40 Communalities (sum of the squared factor loadings) provide an estimation of the proportion of total variance explained for each item by the final model.
Construct validity, known group comparisons. It was hypothesised that: (i) MyPOS symptom and function subscale scores would be higher (representing worse QoL) in patients with poorer ECOG performance status; and; (ii) that the MyPOS symptom and function subscale scores would be higher in patients receiving active treatment compared to those on observation, maintenance or palliative treatment. To account for non‐normally distributed data, parametric (ANOVA) and non‐parametric (Kruksal Wallis) tests were run in parallel. Post hoc, pair‐wise T‐tests, with Bonferroni adjustment, were used to explore differences in means between each group.
Construct validity, convergent and divergent validity. A priori hypotheses about the nature of correlations between the MyPOS total score, MyPOS subscale scores and subscales from the EORTC‐QLQ‐C30 and FACT‐LYM were specified, using Pearson product‐moment correlation coefficients with missing data excluded pairwise. Strong and moderate correlations were predefined as >0.70 and >0.50, respectively. It was hypothesised that: (i) MyPOS total scores would have a strong to moderate negative correlation with the EORTC‐QLQ‐C30 Global Health Status scale; (ii) MyPOS symptoms and function subscale would have a strong to moderate negative correlation with the EORTC‐QLQ‐C30 Physical, Role, Cognitive and Social subscales, and the FACT‐LYM Physical, Functional and Additional Social Well‐being subscales; (iii) MyPOS emotional response subscale would have a strong to moderate negative correlation with the EORTC‐QLQ‐C30 Emotional subscale, and the FACT‐LYM Emotional and Additional Well‐Being subscales; and (iv) MyPOS healthcare support subscale would not correlate strongly with any of the EORTC‐QLQ‐C30 or FACT‐LYM subscales as these measures do not contain items on satisfaction.
Content validity. Content analysis of the MyPOS open questions was used to assess comprehensiveness of the structured items.41 Responses to the opening question “What are your main problems or concerns at the moment?”, and to the second open question “Please list any other symptoms not mentioned above, and tick one box to show how they have affected you over the past week” were analysed to identify symptoms or problems not covered in the MyPOS structured items.
Acceptability and floor/ceiling effects. Acceptability was assessed by the proportion of missing data for each MyPOS item. Floor and ceiling effects were considered present if more than 15% achieved the lowest or highest score.42
Internal consistency. Cronbach's α was determined for MyPOS total scores and MyPOS subscales separately. A α coefficient in the range 0.70‐0.95 was considered desirable to indicate good internal consistency without redundancy of items.41
3. Results
148 patients with FL were screened by clinical teams, seven were ineligible, 12 declined to participate and five withdrew from the study. Sample characteristics are shown in Table 1, and reasons for non‐participation in Fig. S1.
Table 1.
Sample characteristics for cross sectional survey (n=124)
| Age, n, % | |
| <65 | 55 (44.4) |
| ≥65 | 69 (55.6) |
| Median age, range | 66 (27‐94) |
| Gender, n, % | |
| Male | 47 (37.9) |
| Female | 77 (62.1) |
| Marital status, n, % | |
| Single, divorced or separated | 16 (12.9) |
| Married or partnered | 94 (75.8) |
| Widowed | 14 (11.3) |
| Ethnicity, n, % | |
| White British | 113 (91.1) |
| White other | 8 (6.5) |
| Black | 2 (1.6) |
| Other | 1 (0.8) |
| Highest education level, n, % | |
| Primary school | 5 (4.0) |
| Secondary school | 54 (43.5) |
| College/technical qualification | 40 (32.3) |
| University first degree | 18 (14.5) |
| Not known | 7 (5.6) |
| Occupation status, n, % | |
| Working or studying | 31 (25.0) |
| Not working or retired | 18 (14.5) |
| Retired | 75 (60.5) |
| ECOG performance status, n, % (abbreviated descriptions) | |
| 0 ‐ Fully active without restriction | 84 (67.7) |
| 1 ‐ Restricted in physically strenuous activity but ambulatory | 29 (23.4) |
| 2 ‐ Ambulatory but unable to do work activities, Up>50% of waking hours | 7 (5.6) |
| 3 ‐ Limited self‐care, confined to bed/chair>50% of waking hours | 4 (3.2) |
| 4 ‐ Disabled. Cannot carry on any self‐care. Totally confined to bed/chair | 0 (0) |
| Stage at diagnosis, n, % | |
| I Single lymph node group | 17 (13.7) |
| II Multiple lymph node groups on same side of diaphragm | 13 (10.5) |
| III Multiple lymph node groups on both sides of diaphragm | 33 (26.6) |
| IV Multiple extranodal sites or lymph nodes and extranodal disease | 42 (33.9) |
| Missing | 19 (15.3) |
| Treatment status, n, % | |
| Active treatment | 19 (15.3) |
| Maintenance treatment | 31 (25.0) |
| Active monitoring | 54 (43.5) |
| Palliative phase | 20 (16.1) |
| Months since diagnosis, n, % | |
| 0‐12 | 29 (23.4) |
| 13‐24 | 18 (14.5) |
| 25‐36 | 19 (15.3) |
| 37‐48 | 6 (4.8) |
| >48 | 52 (41.9) |
| Median months since diagnosis, range | 34 (1‐328) |
| No. of lines of treatment including current, n, % | |
| 0 | 15 (12.1) |
| 1 | 48 (38.7) |
| 2 | 37 (29.8) |
| 3 | 14 (11.3) |
| More than 3 | 9 (7.3) |
| Missing | 1 (0.8) |
Median age was 66 years old (range: 27‐94). Sixty‐two percent of respondents were women. Most participants (75.8%) were married or partnered, White British (91.1%) ethnic origin, not working or retired (75.0%), and not educated beyond college level (79.8%). Forty‐four percent were managed on active monitoring; 40.3% were receiving active or maintenance treatment; and 16.1% were palliative. Sixty‐eight per cent had ECOG PS 0; 23.4% had ECOG PS 1; 5.6% ECOG PS 2; 3.2% ECOG PS 3; and no participant had ECOG PS 4. Median time from diagnosis was 34 months (IQ range: 25‐84) at the time of questionnaire completion.
3.1. Structural validity: Confirmatory factor analysis (CFA)
Bartlett's test of sphericity (χ2=1525.0, P<.001) and the MSA (0.848) indicated good factorability. Results from the model fit statistics for the three models tested with CFA are presented in Table 2. An iterative improvement in model fit is indicated with each new model. The final model falls short of demonstrating good fit; χ2/df=2.385, CFI=0.774 and RMSEA=0.109 (90% confidence interval: 0.097‐0.121), despite item parcelling increasing the participant to item ratio from 4.5 to 5.1
Table 2.
Model fit index (n=118)
| One factor (no sex; n=118) | Three factor (no sex; n=118) | Three factor (no sex; gastro parcel; n=118) | |
|---|---|---|---|
| No. of items | 26 | 26 | 23 |
| Chi‐square | 968.266 | 710.963 | 541.477 |
| df | 299 | 296 | 227 |
| P‐value | .000 | .000 | .000 |
| Chi‐square/df | 3.238 | 2.402 | 2.385 |
| Comparative fit index (CFI) | 0.567 | 0.732 | 0.774 |
| Root mean squared error of approximation (RMSEA) [90% CI] | 0.138 [0.129‐148] | 0.109 [0.099‐0.120] | 0.109 [0.097‐0.121] |
All items load above 0.30 on the factors predicted (Table 3). ‘Tingling in hands and/or feet’ was close to the lower threshold, with a loading of 0.314, suggesting a weaker correlation between this item and other items on the subscale. Eigenvalue estimates report 54.9% of the total variance explained by the three factors.
Table 3.
Confirmatory factor analysis (Model 3): standardised estimates for item factor loadings and communalities (n=118)
| Symptoms & Function subscale | Emotional response subscale | Healthcare support subscale | Squared multiple correlations (Communalities) | |
|---|---|---|---|---|
| Cronbach's α | .871 | .867 | .749 | |
| Items (abbreviated wording used) | ||||
| 01. Pain | .623 | .388 | ||
| 02. Fatigue or lack of energy | .630 | .398 | ||
| 03. Shortness of breath | .479 | .229 | ||
| 04‐07. Gastro Parcel | .510 | .260 | ||
| 08. Mouth problems | .471 | .222 | ||
| 09. Poor mobility | .737 | .543 | ||
| 10. Tingling in hands or feet | .314 | .098 | ||
| 11. Difficulty remembering | .557 | .311 | ||
| 12. Usual activities | .846 | .717 | ||
| 13. Hobbies and leisure | .847 | .717 | ||
| 14. Quality time with friends | .702 | .493 | ||
| 16. Feeling depressed | .760 | .577 | ||
| 17. Anxious about illness | .818 | .669 | ||
| 18. Worry about infections | .561 | .314 | ||
| 19. Worry about appearance | .651 | .424 | ||
| 20. Worry about finance | .534 | .285 | ||
| 21. Worry illness get worse | .816 | .666 | ||
| 22. Able to cope with illness | .769 | .591 | ||
| 23. Advice if needed | .710 | .504 | ||
| 24. Good knowledge & skill | .890 | .792 | ||
| 25. Show care & respect | .826 | .682 | ||
| 26. Info about illness | .564 | .318 | ||
| 27. Info about future | .457 | .208 | ||
3.2. Construct validity
The first hypothesis of higher MyPOS symptom and function scores in participants with poorer ECOG performance status was confirmed (F=26.17, P<.000). The second hypothesis, poorer MyPOS symptom and function scores in those on active treatment versus those on maintenance, active monitoring and palliative treatment, was also confirmed (F=4.16, P=.008). Figure 1 displays results for the parametric tests for 120 participants who had complete data for the MyPOS symptom and function subscale. Results were confirmed in a sensitivity analysis using non‐parametric tests.
Figure 1.
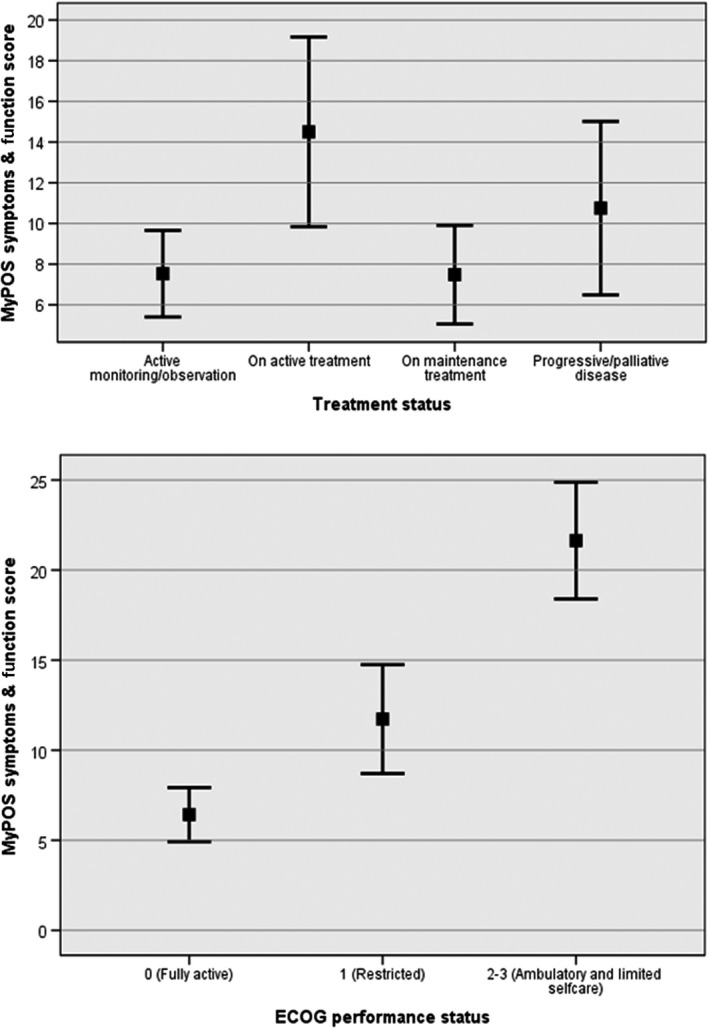
Known group comparisons showing MyPOS symptoms and function subscale scores (mean and 95% CI) by treatment status and ECOG performance status (n=120)
Post‐hoc, pair‐wise comparisons of the subscale means confirmed significant differences between participants on (i) active treatment and maintenance treatment (P=.023); and (ii) active treatment and active monitoring (P=.010), but non‐significant differences between; (iii) active treatment and progressive/palliative disease (P=.891).
3.3. Convergent and divergent validity
All, apart from one, of the hypotheses were confirmed within the predefined limits (for Pearson correlations see Table S2). MyPOS total scores correlated negatively with the EORTC‐QLQ‐C30 Global Health Status scale (r=−.723, P<.01); MyPOS Symptom & Function scores correlated negatively with EORTC‐QLQ‐C30 Physical function (r=−.791, P<.01), Role function (r=−.838, P<.01), Cognitive function (r=−.584, P<.01), and Social function (r=−.634, P<.01). MyPOS Emotional response scores correlated negatively with scores on FACT‐LYM Emotional well‐being (r=−.762, P<.01), Additional concerns (r=−.729, P<.01), and EORTC‐QLQ‐C30 Emotional function (r=−.753, P<.01). Scores on the MyPOS Healthcare support subscale showed no correlation with scores across the subscales from FACT‐LYM and EORTC‐QLQ‐C30 QLQ‐C30, apart from a correlation with the FACT‐LYM Additional Concerns scores exceeding the 0.50 threshold (r=−.518, P<.01).
3.4. Content validity
Eighty‐eight (80%) participants recorded some free text about their main problems or concerns at the beginning of the MyPOS questionnaire. In total, 148 main problems or concerns were reported (Tables S4a‐S4c), with 112 (76%) of these being covered by a subsequent MyPOS item. Of the 36 (24%) main problems and concerns not covered by a MyPOS item, only weight loss was reported multiple times, three separate participants citing it as a main problem or concern.
After grouping, the largest number of concerns (59 (40%)) were about participants worry for their disease or treatment, for example “always wondering when the lymphoma will become active again” and “worried about having chemo again”, captured by MyPOS items 17, 18, 21, 22, and to some extent by items 26 and 27.
A total of 44 additional symptoms were recorded after the symptom list on the questionnaire (Table S5). The majority (35) were unique symptoms recorded only once. Four were about sweating, two of these specifically about night sweats, a further two about problems sleeping, and three referred to swelling in the legs, feet or ankles.
3.5. Acceptability
Ten out of 27 MyPOS items had missing data; most (9) of these were missed by just one or two participants (see Table S1). By far the highest rate of missing data was observed for the question about sex; 13 (10.5%) respondents selected ‘rather not say’ on this item. The proportion of missing data overall was low, 0.7% (23 missing responses out of a possible 3348). Participants used the full range of responses (0‐4) in 19 out of 27 items. For the questions about shortness of breath, vomiting, mouth problems, worry about infections, coping with illness, and the three items about healthcare support, participants used the lower four responses (0‐3) only. Floor effects are evident for all 27 items; the proportion of answers in the lowest category ranged from 23.4% to 91.9%. Ceiling effects were not present for any item.
3.6. Reliability
The MyPOS total and subscale scores showed good internal consistency within the 0.70‐0.95 desired range (Table 3).
4. Discussion
Together with survival, improved HRQOL is a priority for haematological cancer patients.8, 13, 43, 44 Patient‐reported monitoring of HRQOL facilitates clinician‐patient communication about how patients perceive their physical and emotional symptoms, offering the optimal avenue to support patients and effect outcomes.18, 20 A recent randomised control trial of 766 patients with advanced solid tumours has confirmed the clinical benefits associated with patients self‐reporting of symptoms during cancer care, including; ‘fewer ED visits, fewer hospitalisations, longer duration of palliative chemotherapy and superior quality‐adjusted survival’.17
The chronic nature of MM, indolent NHL, and other conditions including, chronic lymphocytic leukaemia (CLL) and chronic myeloid leukaemia (CML), expose patients to the risk of long‐term and accumulated effects of toxicity and adverse psychological effects associated with the pattern of disease relapse and remittance.10, 23 Routine monitoring of HRQOL may be particularly beneficial in the care of these patients, helping to support palliation of symptoms and minimise the cumulative negative effects of treatment and disease on HRQOL.14, 23 Of critical importance to greater integration of HRQOL in the clinical care of haematological cancer patients is the availability of valid and reliable tools, sensitive to specific disease‐related outcomes, yet brief, and acceptable to patients and clinicians.15
This is the first study to demonstrate the validity and reliability of a HRQOL measure for use in patients with FL as a distinct patient group. Although the MyPOS was originally developed for use in patients with myeloma, similarities between the two diseases and a lack of FL‐specific tools, led us to validate the MyPOS in FL. Fatigue, pain, constipation, peripheral neuropathy, dyspnoea, sleep problems, sexual dysfunction, reduced physical and social functioning, and financial problems are common across both patient groups.11, 13, 23 The successful validation of MyPOS in FL supports further development and validation of the tool for use in other indolent NHL conditions and chronic haematological malignancies such as CLL and CML.
Sensitivity to the individualistic nature of QOL is supported in the MyPOS through the inclusion of open text questions. This provides an opportunity for clinicians to discover what matters most to patients, helping to support patient centred care.20 Analysis of the free text recorded by patients established good content validity for MyPOS. Patient's ‘main problems and concerns’ were comprehensively covered. Worry about disease or treatment was prominent, reaffirming the importance of comprehensively capturing these issues in the MyPOS.13, 23 Weight‐loss, problems sleeping, sweating and lower limb swelling are identified as potential areas for extending the symptom list, and should be explored in further qualitative interviews with FL patients.
Despite less than good overall model fit for the final model in the factor analysis, the individual item factor loadings, and the improvement in model fit from the unidimensional to the final model, suggest the three factor model established for MyPOS;24 (i) symptoms and function; (ii) emotional response; (iii) healthcare support, holds for use in FL patients. Model fit, and lower loading of the item about tinging in hands and feet, which has relatively high prevalence in this sample, comparable to that of pain, warrant further exploration in a larger sample. The aligning of items on symptoms and function further supports the theoretical model underpinning the development of the MyPOS, namely the importance of capturing the indirect effect of physical problems on functioning and HRQOL.21, 24
MyPOS performs well as a tool for differentiating between patient groups. In line with existing evidence, patients with lower performance status and those on active treatment had worse symptoms and function.11, 13 Total MyPOS and MyPOS subscale scores also behaved as expected against existing validated measures, the FACT‐LYM and EORTC‐QLQ‐C30. Lack of correlation between the MyPOS healthcare support subscale further supports recognition of these factors as a separate domain within overall HRQOL.
Qualitative evidence supporting the development of MyPOS highlighted the importance patients place on issues pertaining to satisfaction with healthcare staff and information needs.21 Two questions in the original FACT‐G measures,45 and four in the earlier myeloma‐specific version of the EORTC; the MY24,46 related to these issues have since been removed after observing that most patients respond positively. We have similarly observed floor effects for the five items on the MyPOS healthcare support subscale, however, given the importance of these issues in routine clinical care, these items are retained in the MyPOS and further strengthen its use as a clinical tool.
The three dimension model is further supported by the demonstration of good internal consistency with no suggestion of redundancy of items.41
4.1. Methodological limitations
This study is limited by its cross‐sectional design. Validity and reliability, including longitudinal and inter‐rater reliability have been established for the generic Palliative Care Outcome Scale from which the MyPOS is derived,47 and work is currently underway to evaluate the test‐retest reliability and responsiveness of MyPOS in detecting clinically important differences in myeloma patients over time.48 This longitudinal analysis will strengthen our understanding of how to most effectively use MyPOS to improve patient care. Nevertheless, further disease‐specific longitudinal validation of MyPOS in FL patients is needed to support the clinical use of this tool in measuring patient outcomes.
We did not seek access to records to compare our sample to the demographic and clinical characteristics of the wider group of patients registered at participating sites. Consecutive recruitment was used to maximise representativeness, and age and gender distributions reflected those in the wider FL population in the UK.2 However, performance status and length of illness in our sample suggest that the very sickest patients, perhaps because they were non‐ambulatory and confined to their homes, were excluded from our sampling frame. This is reflected in the presence of floor effects in many items. Further investigation of the acceptability of the MyPOS in a less‐well population of FL patients would be beneficial. Moreover, given the original purpose of this tool, for use with myeloma patients, further qualitative work involving in‐depth cognitive interviews with FL patients to evaluate comprehensiveness of the MyPOS items in covering main symptoms and problems in this population is needed.
The MyPOS has previously been demonstrated to be brief, with myeloma patients taking on average (mean) 7 minutes 19 seconds to complete the measure. Information on time taken to complete the MyPOS was recorded by research nurses for only 6 FL patients in this sample and is therefore not reported, this is an omission and should be prioritised in future studies.
Finally, we acknowledge that the confirmatory factor analysis was limited by a small participant to item ratio. Of main concern is that the structural validity of the item concerning worry about sex life has not been established, and the overall fit of the model fell short of predefined thresholds for ‘good fit’, therefore further factor analysis with a larger sample is required.
5. Conclusion
MyPOS, a measure originally developed for use with myeloma patients, is a brief, valid, reliable and acceptable tool for assessing the HRQOL of patients with FL in a clinical setting. Longitudinal validation is now needed to establish test‐retest reliability and responsiveness of the measure to clinical changes over time, to further support the use of the MyPOS in the routine clinical care of FL patients. Furthermore, this work supports the case for validating the MyPOS in similar indolent NHL conditions and other chronic haematological malignancies including CLL and CML.
Authors’ contribution
IJH, SAS, SD and PME won funding for the project and contributed to the conception, design and conduct of the study, with IJH acting as senior researcher. TRO and CR contributed to the study design, collected the data and co‐ordinated the study. CR and JMD planned and conducted the data analysis. JMD prepared the manuscript with all other authors providing comments and critical revisions. The final manuscript was approved by all authors prior to submission.
Conflict of Interest
The authors have no competing interests.
Supporting information
Acknowledgements
We would like to acknowledge our collaborators for their contribution identifying and recruiting participants for the study. Collaborating centres were Bradford Teaching Hospitals NHS Foundation Trust, Colchester Hospital University NHS Foundation Trust, Epsom and St Helier University Hospitals NHS Trust, Frimley Park Hospital NHS Foundation Trust, Maidstone and Tunbridge Wells NHS Trust, Mid Yorkshire Hospitals NHS Trust, Northampton General Hospital NHS Trust, Pennine Acute Hospitals NHS Trust, Surrey and Sussex Healthcare NHS Trust, University Hospitals Coventry and Warwickshire NHS Trust, Weston Area Health NHS Trust and Wye Valley NHS Trust. This work with FL patients was supported by a grant from King's College Hospital NHS Foundation Trust, the development of MyPOS with myeloma patients was supported by Myeloma UK, and St. Christopher's Hospice. Professor Irene J. Higginson is a National Institute of Health Research senior investigator. These collaborating and supporting organisations were not involved in planning the study or preparing the manuscript. The research was supported by Cicely Saunders International and the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South London at King's College Hospital NHS Foundation Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Davies JM, Osborne TR, Edmonds PM, et al. The Myeloma Patient Outcome Scale is the first quality of life tool developed for clinical use and validated in patients with follicular lymphoma. Eur J Haematol. 2017;98:508‐516. https://doi.org/10.1111/ejh.12864
References
- 1. Steliarova‐Foucher E, O'Callaghan M, Ferlay J, et al. European Cancer Observatory: Cancer Incidence, Mortality, Prevalence and Survival in Europe. Version 1.0 (September 2012) European Network of Cancer Registries, International Agency for Research on Cancer. Available from http://eco.iarc.fr. Accessed on November 30, 2016.
- 2. Smith A, Crouch S, Lax S, et al. Lymphoma incidence, survival and prevalence 2004‐2014: sub‐type analyses from the UK's Haematological Malignancy Research Network. Br J Cancer. 2015;112:1575‐1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shankland KR, Armitage JO, Hancock BW. Non‐Hodgkin lymphoma. Lancet. 2012;380:848‐857. [DOI] [PubMed] [Google Scholar]
- 4. Link BK, Maurer MJ, Nowakowski GS, et al. Rates and outcomes of follicular lymphoma transformation in the immunochemotherapy era: a report from the University of Iowa/MayoClinic Specialized Program of Research Excellence Molecular Epidemiology Resource. J Clin Oncol. 2013;31:3272‐3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ansell SM. Follicular lymphoma: watch and wait is watch and worry. Lancet Oncol. 2014;15:368‐369. [DOI] [PubMed] [Google Scholar]
- 6. Bellizzi KM, Rowland JH, Arora NK, Hamilton AS, Miller MF, Aziz NM. Physical activity and quality of life in adult survivors of non‐Hodgkin's lymphoma. J Clin Oncol. 2009;27:960‐966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mols F, Aaronson NK, Vingerhoets AJ, et al. Quality of life among long‐term non‐Hodgkin lymphoma survivors: a population‐based study. Cancer. 2007;109:1659‐1667. [DOI] [PubMed] [Google Scholar]
- 8. Pettengell R, Donatti C, Hoskin P, et al. The impact of follicular lymphoma on health‐related quality of life. Ann Oncol. 2008;19:570‐576. [DOI] [PubMed] [Google Scholar]
- 9. Smith SK, Zimmerman S, Williams CS, Zebrack BJ. Health status and quality of life among non‐Hodgkin lymphoma survivors. Cancer. 2009;115:3312‐3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oerlemans S, Mols F, Nijziel MR, Lybeert M, van de Poll‐Franse LV. The impact of treatment, socio‐demographic and clinical characteristics on health‐related quality of life among Hodgkin's and non‐Hodgkin's lymphoma survivors: a systematic review. Ann Hematol. 2011;90:993‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andresen S, Brandt J, Dietrich S, Memmer ML, Ho AD, Witzens‐Harig M. The impact of high‐dose chemotherapy, autologous stem cell transplant and conventional chemotherapy on quality of life of long‐term survivors with follicular lymphoma. Leuk Lymphoma. 2012;53:386‐393. [DOI] [PubMed] [Google Scholar]
- 12. Cheung MC, Imrie KR, Friedlich J, Buckstein R, Lathia N, Mittmann N. The impact of follicular (FL) and other indolent non‐Hodgkin's lymphomas (NHL) on work productivity‐a preliminary analysis. Psychooncology. 2009;18:554‐559. [DOI] [PubMed] [Google Scholar]
- 13. Oerlemans S, Issa DE, van den Broek EC, et al. Impact of therapy and disease‐related symptoms on health‐related quality of life in patients with follicular lymphoma: results of the population‐based PHAROS‐registry. Eur J Haematol. 2014;93:229‐238. [DOI] [PubMed] [Google Scholar]
- 14. Arden‐Close E, Pacey A, Eiser C. Health‐related quality of life in survivors of lymphoma: a systematic review and methodological critique. Leuk Lymphoma. 2010;51:628‐640. [DOI] [PubMed] [Google Scholar]
- 15. Hlubocky FJ, Webster K, Cashy J, Beaumont J, Cella D. The Development and Validation of a Measure of Health‐Related Quality of Life for Non‐Hodgkin's Lymphoma: The Functional Assessment of Cancer Therapy – Lymphoma (FACT‐Lym). Lymphoma. 2013;2013:9. [Google Scholar]
- 16. EORT QOL Module for Chronic Lymphocytic Leukaemia (CLL), Non‐Hodgkin's Lymphoma (NHL) and Hodgkin's Lymphoma (HL). Available from http://groups.eortc.be/qol/eortc-qol-module-chronic-lymphocytic-leukaemia-cll-non-hodgkin%E2%80%99s-lymphoma-nhl-and-hodgkin%E2%80%99s-lymphoma. Accessed November 31, 2016.
- 17. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient‐reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34:557‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Basch E, Abernethy AP. Commentary: encouraging clinicians to incorporate longitudinal patient‐reported symptoms in routine clinical practice. J Oncol Pract. 2011;7:23‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bush N, Donaldson G, Moinpour C, et al. Development, feasibility and compliance of a web‐based system for very frequent QOL and symptom home self‐assessment after hematopoietic stem cell transplantation. Qual Life Res. 2005;14:77‐93. [DOI] [PubMed] [Google Scholar]
- 20. Higginson IJ, Carr AJ. Measuring quality of life: using quality of life measures in the clinical setting. BMJ. 2001;322:1297‐1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Osborne TR, Ramsenthaler C, de Wolf‐Linder S, et al. Understanding what matters most to people with multiple myeloma: a qualitative study of views on quality of life. BMC Cancer. 2014;14:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ramsenthaler C, Osborne TR, Gao W, et al. The impact of disease‐related symptoms and palliative care concerns on health‐related quality of life in multiple myeloma: a multi‐centre study. BMC Cancer. 2016;16:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ramsenthaler C, Kane P, Gao W, et al. Prevalence of symptoms in patients with multiple myeloma: a systematic review and meta‐analysis. Eur J Haematol. 2016;95:416‐429. [DOI] [PubMed] [Google Scholar]
- 24. Osborne TR, Ramsenthaler C, Schey SA, Siegert RJ, Edmonds PM, Higginson IJ. Improving the assessment of quality of life in the clinical care of myeloma patients: the development and validation of the Myeloma Patient Outcome Scale (MyPOS). BMC Cancer. 2015;15:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Georgakopoulos A, Kontodimopoulos N, Chatziioannou S, Niakas D. EORTC QLQ‐C30 and FACT‐Lym for the assessment of health‐related quality of life of newly diagnosed lymphoma patients undergoing chemotherapy. Eur J Oncol Nurs. 2013;17:849‐855. [DOI] [PubMed] [Google Scholar]
- 26. Cella D, Webster K, Cashy J, et al. Development of a Measure of Health‐Related Quality of Life for Non‐Hodgkin's Lymphoma Clinical Research: The Functional Assessment of Cancer Therapy – Lymphoma (FACT‐Lym). Blood. 2005;106:750. [Google Scholar]
- 27. Yost KJ, Thompson CA, Eton DT, et al. The Functional Assessment of Cancer Therapy – General (FACT‐G) is valid for monitoring quality of life in patients with non‐Hodgkin lymphoma. Leuk Lymphoma. 2013;54:290‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fayers PM, Bjordal K, Curran D, Groenvold M. EORTC QLQ‐C30 Scoring Manual (Third edition). Brussels: EORTC Quality of Life Group; 2001. [Google Scholar]
- 29. Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol. 1989;7:1630‐1636. [DOI] [PubMed] [Google Scholar]
- 30. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982; 5:649‐655. [PubMed] [Google Scholar]
- 31. SPSS. IBM Corp . Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp; 2013. [Google Scholar]
- 32. Arbuckle JL. IBM SPSS Amos 22 User's Guide. US: IBM; 2013. [Google Scholar]
- 33. Little TD, Cunningham WA, Shahar G, Widaman KF. To parcel or not to parcel: exploring the question, weighing the merits. Struct Equ Model. 2002;9:151‐173. [Google Scholar]
- 34. Norman GR, Streiner D. Biostatistics: The Bare Essentials. Hamilton, ON: PMPH ‐ USA; 2008. [Google Scholar]
- 35. Bartlett M. Tests of significance in factor analaysis. Br J Math Stat Psychol. 1950;3:77‐85. [Google Scholar]
- 36. Kaiser H. A second generation little jiffy. Psychometrika. 1970;35:401‐415. [Google Scholar]
- 37. Ullman JB. Structural equation modelling In: Tabachnick BG, Fidell LS, eds. Using Multivariate Statistics. Boston: Ally & Bacon, 2001:676‐780. [Google Scholar]
- 38. Lt H, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Eq Model Multi J. 1999;6:1‐55. [Google Scholar]
- 39. MacCallum RC, Austin JT. Applications of structural equation modeling in psychological research. Annu Rev Psychol. 2000;51:201‐226. [DOI] [PubMed] [Google Scholar]
- 40. Tabachnick BG, Fidell L. Using Multivariate Statistics (Sixth Edition). Edinburgh: Pearson Education Limited; 2014. [Google Scholar]
- 41. Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60:34‐42. [DOI] [PubMed] [Google Scholar]
- 42. McHorney CA, Tarlov AR. Individual‐patient monitoring in clinical practice: are available health status surveys adequate? Qual Life Res. 1995;4:293‐307. [DOI] [PubMed] [Google Scholar]
- 43. Osborne TR, Ramsenthaler C, Siegert RJ, Edmonds PM, Schey SA, Higginson IJ. What issues matter most to people with multiple myeloma and how well are we measuring them? A systematic review of quality of life tools. Eur J Haematol. 2012;89:437‐457. [DOI] [PubMed] [Google Scholar]
- 44. American Society of Clinical Oncology . Outcomes of cancer treatment for technology assessment and cancer treatment guidelines. J Clin Oncol 1996;14:671‐679. [DOI] [PubMed] [Google Scholar]
- 45. Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570‐579. [DOI] [PubMed] [Google Scholar]
- 46. Stead ML, Brown JM, Velikova G, et al. Development of an EORTC questionnaire module to be used in health‐related quality‐of‐life assessment for patients with multiple myeloma. European Organization for Research and Treatment of Cancer Study Group on Quality of Life. Br J Haematol. 1999;104:605‐611. [DOI] [PubMed] [Google Scholar]
- 47. Costantini M, Rabitti E, Beccaro M, et al. Validity, reliability and responsiveness to change of the Italian palliative care outcome scale: a multicenter study of advanced cancer patients. BMC Palliat Care. 2016;15:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ramsenthaler C, Osborne TR, de Wolf‐Linder S, et al. The Myeloma Patient Outcome Scale (MyPOS) – Longitudinal validity and reliability of a measure of quality of life for clinical use in patients with multiple myeloma. Eur J Palliat Care. 2015;22:19. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


