Figure 4.
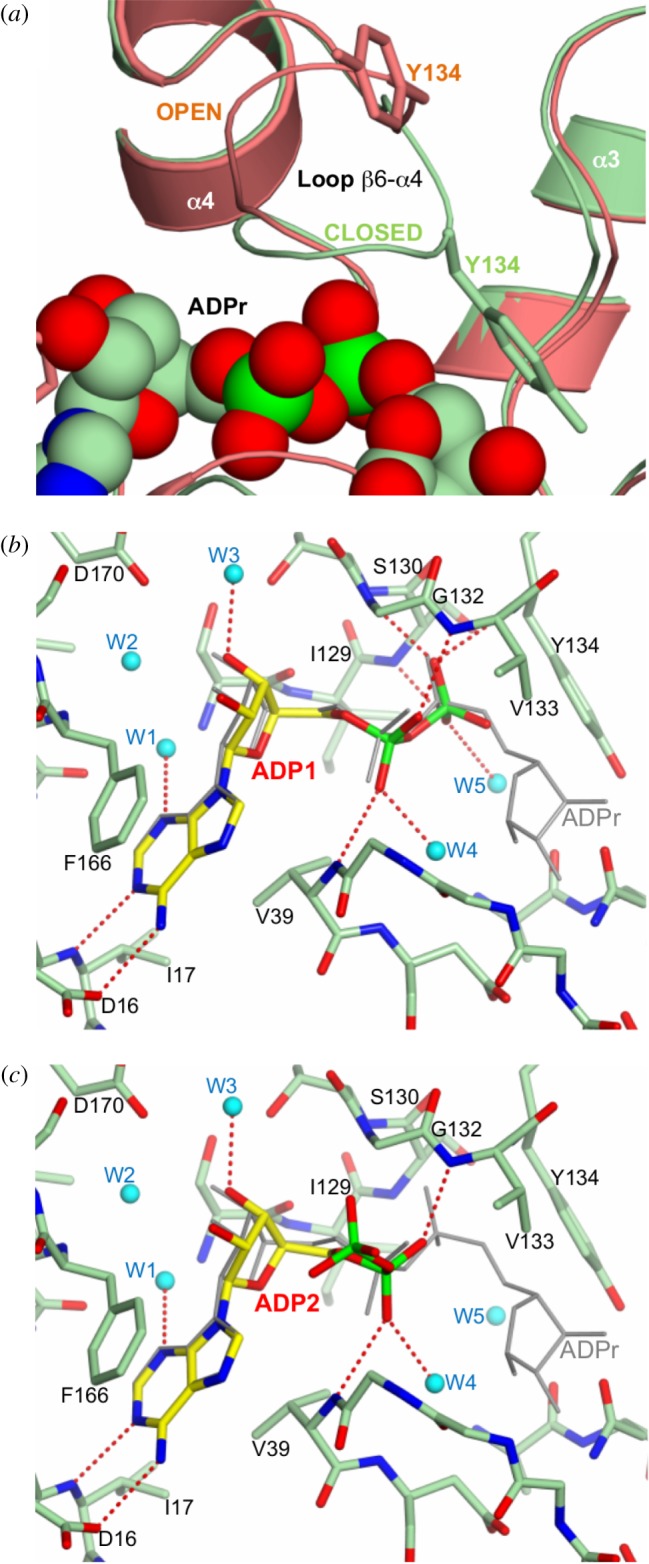
Substrate-induced changes during ADPr binding. (a) Superimposition of the OiMacroD-ADPr (green) and D40A structures (salmon), highlighting the closure of the β6–α4 loop induced upon ADPr binding with the concomitant movement of Y134. (b,c) Interactions established by the two alternative conformations of the ADP molecule (Cα in yellow) observed in the OiMacroD-ADP structure. The ADPr (grey) found in OiMacroD-ADPr structure was superimposed. Water molecules are represented as spheres (cyan).
