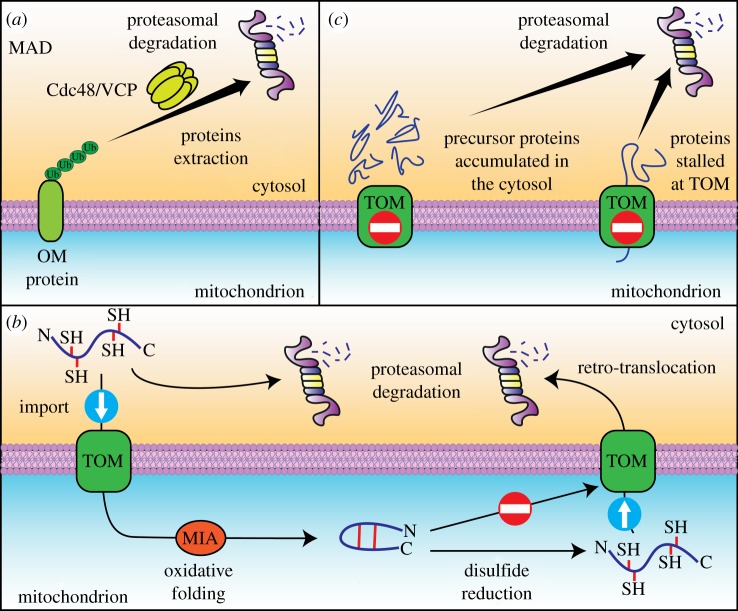
Figure 3.
Proteasome-dependent strategies of mitochondrial protein degradation. Mitochondrial proteins are exposed to the proteasomal degradation in several ways. (a) The mitochondria-associated protein degradation (MAD) process is homologous to endoplasmic reticulum-associated protein degradation (ERAD). During MAD, mitochondrial outer membrane (OM) proteins are extracted from the organelle through the highly conserved AAA-ATPase Cdc48 (yeast; VCP or p97 in mammals) and directed to proteasomal degradation. (b) The proteasomal degradation of mitochondrial proteins also occurs after protein retro-translocation from the mitochondrial intermembrane space. Once proteins are translocated to the mitochondrion through the TOM complex, they are trapped inside the organelle as a result of their oxidative folding that is orchestrated by the mitochondrial import and assembly (MIA) machinery. If the disulfide bonds are not formed or become reduced, then the protein can retro-translocate and be degraded by the proteasome in the cytosol. (c) The proteasome is also involved in the degradation of mitochondrial precursor proteins that fail to be transported to the organelle because of defects in protein import or protein stalling on the translocase of the outer membrane (TOM) complex.