Abstract
In the post-genomic era, the goal of personalized medicine is to determine the correlation between genotype and phenotype. Developing high-throughput genotyping technologies such as genome-wide association studies (GWAS) and the 1000 Genomes Project (http://www.internationalgenome.org/about/#1000G_PROJECT) has dramatically enhanced our ability to map where changes in the genome occur on a population level by identifying millions of single nucleotide polymorphisms (SNPs). Polymorphisms, particularly those within the coding regions of proteins and at splice junctions, have received the most attention, but it is also now clear that polymorphisms in the non-coding regions are important. In these non-coding regions, the enhancer and promoter regions have received the most attention, whereas the 3′-UTR regions have until recently been overlooked. In this review, we examine how SNPs affect microRNA-binding sites in these regions, and how mRNA stability changes can lead to disease pathogenesis.
Keywords: poly-miRTS, microRNA, single nucleotide polymorphisms
1. Introduction
Single nucleotide polymorphisms (SNPs) occur in 1% or more within the population [1]. Although these populations are identical in 99.5% at the DNA level [2], there are approximately 10 million SNPs in the human genome, indicating that they occur once in every 300 bp in both coding and non-coding regions of genes [3]. SNPs in the coding region can result in synonymous and non-synonymous changes, with the latter resulting in an amino acid change or the introduction of a stop codon [4]. These changes can lead to human diseases [5], and in fact at least 25% of the reported non-synonymous SNPs are predicted to negatively affect protein function [6,7].
Synonymous SNPs have been referred to as silent mutations because they do not change the amino acid [8]. However, there is a growing body of evidence indicating that synonymous SNPs do affect proper protein function [9]. For example, two synonymous SNPs in the sequence encoding the multidrug resistance protein 1 (MDR1) affect protein folding and function [10]. Moreover, the most common disease-causing mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene is an out-of-frame deletion of phenylalanine-508 (ΔF508) that introduces a SNP at isoleucine-507 (I507) and this SNP contributes to the severity of the ΔF508 CFTR channel dysfunction [11,12].
Recently, more attention has been paid to the SNPs identified in non-coding regions. Interestingly, about 93% of functional SNPs in the GWAS catalogue are in non-coding regions [13]. They have been called regulatory SNPs (rSNPs) because they affect transcriptional regulation or post-transcriptional gene expression [14]. rSNPs can cause changes in cell function at different levels of gene regulation. For example, they can affect gene splicing [15] and transcription factor binding [16]. These rSNPs reside in the sequence of non-coding RNA in the promoter and enhancer regions [16]. They can also affect the half-life of messenger RNA (mRNA) and result in decreased protein levels through mRNA–microRNA (miRNA) interactions. SNPs in miRNA target sites in the 3′-UTR of mRNAs are referred to as poly-miRTSs [17]. The SNP dataset from the UCSC Genome browser (NCBI dbSNP, Build 130 [18]) consists of 18 833 531 human SNPs, while the genomic coordinates for a subset of 175 351 (approx. 11%) locates them in the 3'-UTRs of 16 810 genes [19]. Given that there are an estimated 19 000–20 000 genes in the human genome, this suggests that the majority of the genes could be regulated by miRNAs [20], indicating that the potential biological consequence of these mutations should be carefully considered. Furthermore, a substantial number of SNPs and rare mutations within pri-, pre- and mature miRNA sequences have been reported [21,22]. Although some of these miRNA SNPs are related to human diseases [23–27] (reviewed in [17]), their biological role is difficult to elucidate given that changes in any miRNA can have profound genome-wide effects since miRNAs can bind to hundreds of different mRNAs. Since 2008, when Sethupathy & Collins [17] critically reviewed reports of miRNA SNPs involved in human diseases and provided clear criteria for validation of such associations, a large number of novel human disease-related poly-miRTSs have been proposed. Furthermore, recently developed approaches dedicated to miRNA function, targeted genome editing with in silico methods provide novel tools for complex verification of miRNA SNP consequences. In this review, we focus on poly-miRTSs and their potential impact in human diseases.
2. SNPs in miRNA target sites
2.1. mRNA : miRNA association
miRNAs are short (approx. 22 nt) endogenous non-coding single-stranded RNAs which act as post-transcriptional regulators of gene expression [28]. In the cytosol, mature miRNAs that are a part of the Argonaute-containing silencing complexes called miRNA ribonucleoprotein complexes (miRNP) can downregulate a specific target mRNA by Argonaute-catalysed degradation of the mRNA target strand in P bodies or by translational repression [29,30]. Hence, the major consequence of miRNA : mRNA pairing is loss of protein expression, resulting from either decreased transcript levels or by translational repression [29].
Although the mechanism underlying the recognition of mRNA targets by miRNAs has been extensively studied, the minimal requirements for a functional mRNA : miRNA association are not fully understood. Furthermore, despite the fact that many mRNAs have conserved miRNA target sites, a variety of interactions through non-conserved sites has been reported [31]. Finally, the average size of the human 3′-UTR is about 950 nt (for highly expressed neuronal genes it is 1300 nt, whereas for genes specific to non-neuronal tissue it is only 700 nt) [32], while the efficient miRNA-binding site consists of 6–8 nt. Hence, the 3′-UTR of a specific mRNA can include tandem target sequences for a specific miRNA as well as target sequences for many other miRNAs. The composition of specific miRNAs associated with the 3′-UTR of a mRNA along with the efficiency of miRNA pairing to their target sequences impacts the mRNA's half-life and influences protein levels [33,34]. Hence, determining the consequences of SNPs in miRNA target sites is not a trivial endeavour.
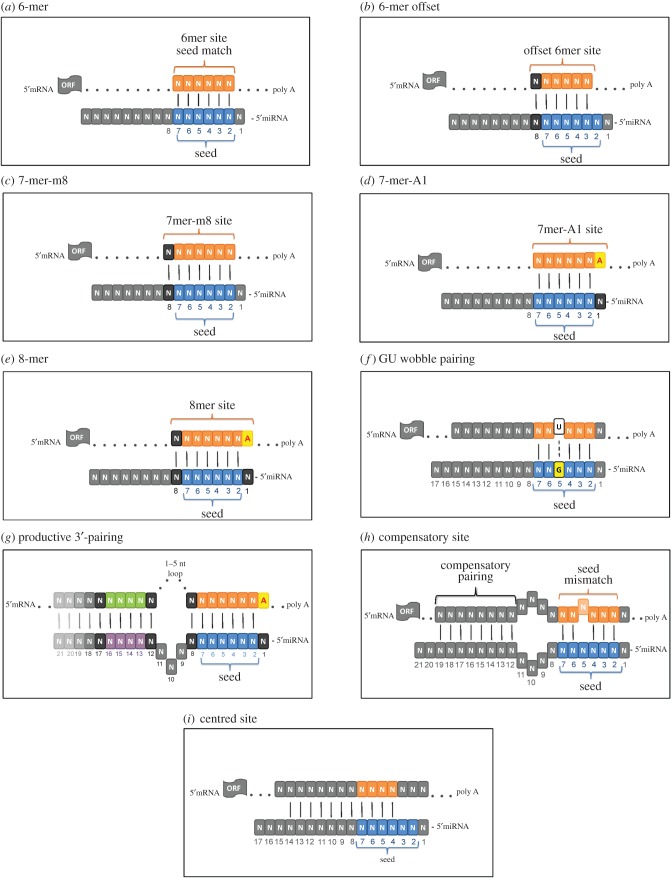
That being said, it is well established that the complementary pairing of a 3′-UTR of a mRNA to a conserved heptametrical seed sequence is usually found at positions 2–7 from the miRNA 5′-end and is critical for mRNA target selection [35]. Initially, it was thought that perfect complementarity of the 3′-UTR of a mRNA to the miRNA seed sequence led to transcript degradation, and a partial match caused translational inhibition [35]. However, recent studies have shown that non-canonical sites also exist and can regulate mRNA degradation [36]. Furthermore, base pairing between mRNA and miRNA seed sequences do not always lead to decreased expression of target transcript [37]. The above findings suggest that additional features of mRNA target sequences play a crucial role in effective miRNA binding. The detailed analysis of seed sequences established 8-nt pairing (8-mer) with mRNA as the most effective, whereas 7- and 6-nt binding sites (7-mer and 6-mer) were less effective (figure 1). Although 6-mers often provide efficient pairing, even in an offset position (figure 1a,b), a 4-mer is a non-functional site in vivo [38]. Interestingly, 7-mer pairing efficiency relies strictly on sequence complementarity. Consequently, although the 7-mer-m8 site (an exact match to positions 2–8 of the mature miRNA—the seed and position 8 (figure 1c)) has increased seed pairing compared with the 6-mer, the 7-mer-A1 (an exact match to positions 2–7 of the mature miRNA—the seed followed by an ‘A1’) has similar seed pairing to 6-mer (figure 1d). The seed pairing including both the match at position m8 and the A1 is characteristic for a 8-mer site [37] (figure 1e). The effect of G : U base pairs and bulges in the seed were also considered showing that a single G : U wobble or target sites with a 1 nt bulge can still be functional [38] (figure 1f). However, the Watson–Crick base pairing is absolutely critical between nucleotides at positions 9–12 in the target site, since the hydrolysis of the phosphodiester backbone in mRNA cleaved by miRNA occurs only when the 10th and 11th nucleotides of mRNA are complementary to nucleotides at positions 2–15 in miRNA [39].
Figure 1.
Types of mRNA : miRNA interactions. (a) 6-mer, (b) 6-mer offset, (c) 7-mer-m8, (d) 7-mer-A1, (e) 8-mer, (f) GU wobble pairing, (g) productive 3'-pairing, (h) compensatory site and (i) centred site.
Furthermore, additional mRNA pairing to the 3′ region of miRNA, termed as productive seed pairing, can increase the target recognition or it can compensate for the mismatch to the seed (3′ supplementary sites and 3′ compensatory sites, respectively) [36]. The substantial pairing of 3′ compensatory sites to mRNA increases the weak 5′ pairing, resulting in functional miRNA binding (figure 1g,h).
Interestingly, Shin et al. [30] indicated that centred mRNA sequences consisting of 11 nt create Watson–Crick pairs with miRNA nucleotides at positions 4–14 or 5–15 and serve as a type of miRNA target site. This unique class of miRNA target sites is devoid of both perfect seed pairing and 3′ compensatory pairing but can be supplemented by pairing to the other miRNA areas (figure 1i).
Based on the studies discussed above, mRNA target sites can be divided into two major groups. The first group consists of canonical sites with (i) strong seed pairing to the 5′ end of miRNA (low pairing energy) that is amplified through either strong base pairing to the 3′ end of the miRNA (an extension of the seed type) or (ii) strong seed pairing to the 5′ end of miRNA seed sites requiring little or no 3′-UTR pairing support. These canonical sites have pairing energy and are often functional in one copy. In contrast with these sites, the second groups are non-canonical seed sites with higher pairing energy that should exist in the 3′-UTR in more than one copy to be effective [38]. It has to be stressed that the seed region contributes the majority of binding energy and strong binding relies mainly on base pairing within this region, whereas an additional 3′ pairing only slightly reduces binding energy [40]. Interestingly, pairing beyond position 16 and at positions 10–11 increases binding energy that results in weakened binding [40].
Another factor to consider in miRNA : mRNA interactions is the location of the target mRNA sites. In general, the 3′-UTR mRNA sites are most efficient [37,41]. Furthermore, target mRNA sites positioned within at least 15 nt from the stop codon, sites located away from the centres of long 3′-UTR, as well as those miRNA target sites located in AU-rich regions are the most effective [37,41]. Additionally, a location of target mRNA sites in close proximity to protein-binding sites and to other miRNA-binding sites may also affect miRNA : mRNA associations [33,37]. The 3′-UTR mRNA quartiles bordering the mRNA poly(A) tail and the ORF exhibit more effective targeting than remaining two centred quartiles. However, this effect was apparent only for longer 3′-UTRs (more than 1300 nt) [37].
Taking into account the complexity of miRNA : mRNA pairing, the introduction of a SNP into a 3′-UTR can have numerous functional consequences by either introducing or removing miRNA target sequences or changing the binding efficiency. The poly-miRTSs within the canonical seed sequence can either create a novel mRNA target site from a preexisting 5-mer sequence (into 6-mer offset or 6-mer) or impair the existing target site 6-mer or 6-mer offset sequence (into 5-mer). Furthermore, since the introduction of poly-miRTSs into seed regions can also affect miRNA : mRNA binding efficiency, it can lead to either increased or decreased post-transcriptional mRNA regulation. Finally, poly-miRTSs may also affect miRNA-binding efficiency by changing supplemental seed pairing that applies to both canonical and non-canonical binding sites. Additionally, in the case of non-canonical binding sites, poly-miRTSs may introduce or remove tandem target sites, and thus change the miRNA effects. Finally, the introduction or removal of miRNA target sites may affect binding to other miRNA target sequences in the SNP's close proximity, which could have unforeseen effects on the mRNA half-life. Given the number of SNPs in the human population, it is not surprising that poly-miRTSs have been shown to affect the levels of numerous proteins that have been associated with various disorders (table 1) [39]. Below, we discuss examples of several studies identifying poly-miRTSs and their potential association with human disorders.
Table 1.
Reports of poly-miRTS associations with human disease. Bold indicates the studies fulfilled the criteria for assigning SNPs as poly-miRTSs involved in human diseases and included: (i) functional experimental validation of SNPs related to differential mRNA targeting; (ii) genetic testing of the association with the disease that takes into account the effects of population stratification; and (iii) mechanistic testing underlying the mechanism by which poly-miRTSs contribute to the disease [17].
| associated disease or trait | miRNA | target gene | putative risk allele | functional association test for allele-specific effects on miRNA targeting | association test | population | refs |
|---|---|---|---|---|---|---|---|
| small cell lung cancer SCLC | miR-191, miR-887-3p | MDM4 | rs4245739 A>C (C creates a new binding site) | in vitro: reporter gene assay in SCLCH446 cells (with miR mimics, or negative controls). | yes | Han Chinese | [42] |
| prostate cancer | miR-191, miR-887-3p | MDM4 | rs4245739 A>C (C creates a new binding site) | in vitro: reporter gene assay in PC3 cells (with miR mimics, or negative controls). | none | — | [43] |
| ovarian cancer | miR-191 | MDM4 | rs4245739 A>C (C creates a new binding site) | in vitro: reporter gene assay in A2780 cells (with miR mimics). Target site and mismatch control blocker were used. | yes | Caucasian women | [44] |
| non-Hodgkin lymphoma NHL | miR-191 | MDM4 | rs4245739 A>C (C creates a new binding site) | none | yes | Han Chinese | [45] |
| oesophageal squamous cell carcinoma ESCC | miR-191 | MDM4 | rs4245739 A>C (C creates a new binding site) | none | yes | Han Chinese | [46] |
| non-small cell lung cancer NSCLC | miR-887-3p | MDM4 | rs4245739 A>C (C creates a new binding site) | in vitro: reporter gene assay in A549 cells (with miR mimics, or negative controls). | yes | Chinese | [47] |
| bladder cancer | miR-140-5p | TP63 | rs35592567 C>T (T creates a new binding site) | in vitro: reporter gene assay in T24, EJ, 5637, J82 and 293A cells (with miR mimic or control). | yes | Han Chinese | [48] |
| type 2 diabetes mellitus T2DM | miR-214-5p, miR-550a-5p | HNF1B | rs2229295 C>A (A creates a new binding site) | in vitro: reporter gene assay in HEK293 cells (with miR mimics). | yes | Japanese | [49] |
| coronary heart disease | miR-4271 | APOC3 | rs4225 G>T (T creates a new binding site) | in vitro: reporter gene assay in 293T and HepG2 cells (with miR mimic, inhibitor or control). | yes | Han Chinese | [50] |
| hypertriglyceridaemia | miR-485-5p | APOA5 | c.a*158C>T rs2266788 (rare c.a*158C allele creates a new binding site) | in vitro: luciferase expression vectors in 293T cells (with miR mimic, inhibitor or control); luciferase expression vectors in HuH-7 cells–investigating endogenous miR functionality (with miR inhibitor or control). | none | — | [51] |
| antropometrics (obesity related phenotype) | miR-522 | PLIN4 | rs8887 G>A (A creates a new binding site) | in vitro: luciferase expression vectors in COS7 cells (with miR mimic or control). | yes | Genetics of Lipid Lowering Drugs and Diet Network (GOLDN) and the Framingham Offspring Study (FOS). | [52] |
| Friedreich's ataxia FRDA | miR-124-3p | FXN | rs11145043 G>T (T creates a new binding site) | in vitro: reporter gene assay in HEK-293 cells (with miR mimic, or mimic negative control). Plasmid constructs differed in more than one SNP. | yes | paediatric cases (the Necker Children's Hospital), adult cases (the CHR Félix Guyon, Saint-Denis, La Réunion, France), controls (patients genetically tested at the Necker Children's Hospital for diseases non-related to FRDA). | [53] |
| Parkinson's disease | miR-34b | SNCA | rs10024743 T>G (unspecified) | in vitro: reporter gene assay in SH-SY5Y human neuroblastoma cells (with pre-miR or miR inhibitor; internal control). Immunocytochemistry using an anti-α-syn antibody. | none | — | [54] |
| breast cancer | miR-96, miR-182 | PALLD | rs1071738 C>G (G impairs binding site) | in vitro: reporter gene assay in HeLa and HEK-293T cells (with miR mimic, miR inhibitor or control). | yes | 460 homogeneous samples (Caucasians); study-sample: 68% Caucasians, 16% African-American, 6% Asian and 10% others | [55] |
| schizophrenia | miR-137 | EFNB2 | rs550067317 A>C (C impairs binding site) | in vitro: reporter gene assay in HEK293T and SH-SY5Y cells (with miR mimic or negative control). | none | Han Chinese | [56] |
| pancreatic ductal adenocarcinoma | miR-199a | HIF1A | rs2057482 T>C (C impairs binding site) | in vitro: reporter gene assay in HEK293T and Panc-1 cells (with miR mimic, miR inhibitor or inhibitor control) In vivo: HIF-1 expression in PDAC tissues (different genotypes). | yes | Han Chinese | [57] |
| bladder cancer | miR-27b | DROSHA | rs10719 T>C (C impairs binding site) | in vitro: reporter gene assay in T24 and J82 cells (with miR mimic, stable negative control, miR inhibitor or inhibitor negative control), In vivo: analysis of total RNA in 61 bladder tumour tissues with different genotypes (32 for TT, 24 for TC, and 5 for CC). | yes | Han Chinese in Beijing (CHB) | [58] |
| Parkinson's disease | miR-433 | FGF20 | rs12720208 C/T (T impairs binding efficiency) | in vitro: reporter gene assay in Neuro2A cells (with miRNA mimic) In vivo: immunoblot analysis in three human brains with different genotypes. | yes | white Americans | [59] |
| Tourette's syndrome | miR-189 | SLITRK1 | var321-SLITRK1 G>A (A creates Watson–Crick pairing instead of G:U wobble base pairing) | in vitro: reporter gene assay in Neuro2A mouse neuroblastoma cells (with miR mimic or control). | yes | more than 80% white | [60] |
| hereditary spastic paraplegia type 31 | miR-140 | REEP1 | c.606+50G>A (A impairs G:U wobble base pairing) | in silico: miRNA target prediction program. | none | of European descent | [61] |
| hereditary spastic paraplegia type 31 | miR-140 | REEP1 | c.606+43G>T (T impairs G:U wobble base pairing) | in silico: miRNA target prediction program. | none | of European descent | [61,62] |
| hereditary spastic paraplegia type 31 | miR-691 | REEP1 | c.606+14C>T (unspecified) | in silico: miRNA target prediction program. | none | of European descent | [62] |
| breast cancer | miR-206 | ESR1 | rs9341070 C>T (T allows more effective binding) | in vitro: reporter gene assay in MCF-7 breast cancer cells (with pre-miR-206, miR inhibitor or let-7 specific modified RNA). | none | — | [63] |
| hypertension | miR-155 | AGTR1 | rs5186 A>C (C impairs binding site) | in vitro: reporter gene assay in 293T cells (with miR mimic or let-7c). | yes | [64] | [64] |
| methotrexate resistance | miR-24 | DHFR | rs34764978 C>T (T impairs binding efficiency) | in vitro: reporter gene assay in DG44 CHO cells (with miR mimic, miR inhibitor, positive or negative control). | none | — | [65] |
| childhood asthma | miR-148a, miR-148b, miR-152 | HLA-G | rs1063320 C>G (G creates a new binding site) | in vitro: reporter gene assay in JEG3 cells (with miR mimic, positive or negative control). | yes | white Americans | [66] |
| arson or property damage | miR-96 | HTR1B | rs13212041 [A/G] (G creates G:U wobble base pairing instead of Watson–Crick base pairing) | in vitro: reporter gene assay in HeLa cells (with miR mimic). | yes | white college students | [67] |
| colorectal cancer | miR-337, miR-582, miR-200a-5p, miR-184, miR-212 | CD86 | rs17281995 G>C (for miR-337, miR-582, and miR-200a-5p, C impairs binding efficiency; for miR-184 and miR-212, C increases binding efficiency) | in silico: miRNA target prediction program. | yes | from Czech republic | [68] |
| colorectal cancer | miR-612 | INSR | rs1051690 G/A (unspecified) | In silico: miRNA target prediction program. | yes | from Czech republic | [68] |
| diarrhoea predominant irritable bowel syndrome | miR-510 | HTR3E | rs56109847 (previously rs62625044) G>A (A impairs binding site) | in vitro: reporter gene assay in HEK293 and Colo320 cells (with miR precursor, miR inhibitor or negative control). | yes | British | [69] |
2.2. Creation of new miRNA target sites by SNPs
2.2.1. MDM4 | miR-191 or miR-877-3p
Mdm2-like p53-binding protein (MDM4) is an oncoprotein that negatively regulates the p53 tumour suppressor protein [70]. It is well documented that overexpression of this protein leads to cancer development [70]. Recent studies suggested that the variation in the 3'-UTR of MDM4 can lead to a decreased risk of various malignancies [42–47]. The occurrence of the C minor allele (SNP rs4245739 A>C) in the 3'-UTR of MDM4 has been shown to decrease the risk of cancer, and delay the progression of metastasis and cancer-related death [42–47]. Numerous studies have shown that introduction of this C minor SNP creates a new binding site for miR-191 [42–46] and/or miR-887-3p [42,43,47], and this leads to a decreased level of MDM4 protein. Moreover, a recently conducted meta-analysis of 69 477 subjects (19 796 cases of nine various type of cancer and 49 681 controls) showed that the above-mentioned SNP correlates with a reduced overall risk of cancer [71].
2.2.2. ΔNp63 | miR-140-5p
p63 is another tumour suppressor protein belonging to the p53 family. Because of different promoters and alternative splicing, there are two major isoforms of TP63: TAp63 (acidic transactivation domain present) and ΔNp63 (no transactivation domain) [72]. Interestingly, in vivo experiments indicate that TAp63 acts like a tumour suppressor gene, whereas ΔNp63 is an oncogene [73–75]. Wang et al. [48] found that the SNP rs35592567 (C>T) in the 3′-UTR of ΔNp63 has an impact on bladder cancer risk. Analysis showed that the T allele is correlated with a decreased risk of bladder cancer because miR-140-5p is able to bind to the 3'-UTR of ΔNp63. Overexpression of miR-140-5p in 5637 cells (urinary bladder grade II carcinoma cells) attenuated cell migration and invasion and inhibited cell proliferation [48].
2.2.3. HNF1B | miR-214-5p and miR-550a-5p
Another example of a positive effect of a SNP on a disease risk is rs2229295 (C>A), which is located in the 3′-UTR of hepatocyte nuclear factor 1-beta (HNF1B) mRNA. This gene encodes a transcription factor known to be a regulator of growth and development in the pancreas [76]. Since HNF1B has a role in controlling hepatic insulin activity and glucose metabolism in vivo [77], Goda et al. [49] suggested that the rs2229295 SNP may correlate with susceptibility for type 2 diabetes mellitus (T2DM). Using luciferase reporter vectors, they demonstrated that the A allele constructs were regulated by two miRNAs: miR-214-5p and miR-550a-5p, whereas C allele constructs were not. Hence, the presence of A allele decreases HNF1B protein levels and has a protective effect against T2DM [49].
2.2.4. APOC3 and APOA5 | miR-4271 and miR-485-5p
APOC3 and APOA5 are genes that encode apolipoprotein C3 and A5, respectively. Both of these proteins along with lipoprotein lipase (LPL) and apolipoprotein C2 (APOC2) are involved in triglyceride metabolism [50,51]. Hu et al. [50] demonstrated that decreased levels of APOC3 lead to lower triglyceride levels and reduce the risk of coronary heart disease (CHD). This is due to SNP (rs4225 G>T) found in the 3′-UTR of APOC3. When the T minor allele is present in the cell, miR-4271 is able to bind to the 3′-UTR of APOC3, and this leads to a decreased translation of APOC3. miR-4271, however, cannot bind to the variant containing the G major allele [50]. Similarly, APOA5 c.*158C>T (rs2266788) is also associated with alterations in triglyceride metabolism and results in hypertriglyceridaemia [51]. In this case, the rare c.*158C APOA5 allele creates a new functional binding site for miR-485-5p. Importantly, both miRNAs regulating APOC3 and APOA5 are endogenously expressed in the human liver, so if the SNP occurs, they may be involved in the regulation of triglyceride metabolism in vivo. However, both examples of SNPs and their impact on the risk of disease need further clarification, since different results have been obtained for different ethnic groups [50].
2.2.5. PLIN4 | miR-522
PLIN4 (perilipin 4) is a member of the perilipin family and these proteins coat the intracellular lipid storage droplets (LSD). PLIN4 has been proposed to promote uptake of free fatty acids from the blood to the LSD and is dependent upon the cell's nutritional status [78]. Meta-analysis of two populations of this gene, rs8887 (G>A), analysed with antropometric measurements, indicated that the two populations were different. Individuals with the A minor allele had an increased volume of visceral and subcutaneous adipose tissue, and higher BMI and weight compared to individuals with the G major allele [52]. This study reported that PLIN4 is regulated by miR-522 only in the rs8887A variant. It is not yet clear, however, if the lower expression of PLIN4 contributes to obesity because the results are conflicting [79,80].
2.2.6. FXN | miR-124-3p
Reduced expression of the mitochondrial frataxin (FXN) protein has been postulated to play a role in Friedreich's ataxia (FRDA), an inherited neurodegenerative disease [81]. Lower levels of frataxin are due to GAA repeat expansion in the FXN gene [81]. Additionally, Bandiera et al. [53] have suggested that miR-124-3p regulates FXN expression in vivo only in FRDA patients. They identified seven SNPs in the 3′-UTR of FXN in children and adults diagnosed with FRDA. One of them, rs11145043 (G>T), permits miR-124-3p binding only when the T allele is present. Although miR-124-3p is highly expressed in the nervous system [82], it is overexpressed in FRDA patients [83], suggesting its role in FRDA. However, its influence on FXN needs further clarification.
2.3. Loss of miRNA target sites by SNPs
2.3.1. SCNA | miR-34b
The α-synuclein SCNA gene polymorphism is considered a main risk for the common sporadic form of Parkinson's disease (PD; approx. 90% of all PD cases) [84]. α-Synuclein is a crucial protein that creates immunoreactive aggregates in Lewy-bodies, which are typical for Parkinson's disease patients' brains [85]. Studies have indicated that miR-34b targets the α-synuclein mRNA3′-UTR in two distinct sites and represses translation of this protein [86]. Importantly, in PD patients' brains, the level of miR-34b in the substantia nigra is decreased. Kabaria et al. [54] have identified a SNP, rs10024743 (T>G), in the 3′-UTR of α-synuclein, which is localized in the target site 1 of miR-34b. This SNP diminishes the miR-34b-mediated repression of α-synuclein levels due to disruption of the miRNA : mRNA association. However, this study was performed only on SH-SY5Y cells and its association with PD remains unclear [54].
2.3.2. PALLD | miR-96 and miR-182
The PALLD gene encodes the actin-associated protein Palladin, whose expression correlates closely with the pathological cell motility characteristics of aggressive cancer cells. The expression level of Palladin in breast cancer patients is higher in invasive and malignant cancer cell types than in non-invasive and normal cell lines. The results suggest that Palladin promotes podosome formation, regulates the actin cytoskeleton via multiple pathways, participates in matrix degradation, and thus facilitates metastasis in breast cancer [87,88]. Gilam et al. [55] have reported that miR-96 and miR-182 reduce breast cancer cell migration and invasion by downregulating Palladin protein levels and that this process is disrupted by a SNP, rs1071738 (G < C), located in the 3′-UTR of the PALLD gene. This SNP is characterized by highest minor allele frequency (greater than 43%) and the alternate G allele is much more common than the ancestral minor C allele. If the C allele occurs in the binding site, the mRNA target sequence at the 3′-UTR of PALLD is fully complementary to the miR-96 and miR-182 seed regions, whereas the presence of the alternate G allele results in one mismatch. A significant decrease in Palladin levels is diminished by miR-96 and miR-182 expression (approx. 30% and approx. 70% reduction, respectively) in the presence of the C allele, but not in the presence of the G allele due to the disrupted miRNA:mRNA association. These findings suggest that although miR-96 and miR-182 may prevent breast cancer metastasis, the functional rs1071738 G variant abolishes this effect [55].
2.3.3. EFNB2 | miR-137
The EFNB2 (ephrin-B2) gene encodes an ephrin, a protein tyrosine kinase that is involved in remodelling and the development of synaptic connections that are regulated by activated NMDA receptor. Ephrin-B2 is essential for the Reelin pathway controlling neuronal migration. Additionally, the activation of EFNB2 is crucial for rescuing the Reelin defect and disruption of this pathway is associated with schizophrenia [56,89]. Recently, a negative correlation between miR-137 and EFNB2 expression was shown [56]. Importantly, the SNP rs550067317 (A>C) is located at the predicted target site of miR-137 in the 3′-UTR of EFNB2. The minor C allele of rs550067317 disrupts the formation of the typical stem-loop structure during base pairing of miR-137 with the predicted target sequence at the 3′-UTR, consequently reversing inhibition of EFNB2 expression.
2.3.4. HIF1A | miR-199a
The HIF1A gene encodes the HIF-1α protein (hypoxia-inducible factor 1), an oxygen dependent subunit and master transcriptional regulator of the mammalian cell response to oxygen deprivation, and is therefore important in both the cardiovascular and cancer fields. To date, numerous studies have demonstrated miRNA's role in regulation of HIF-1α levels [90–93]. Recently, a SNP (rs2057482 T>C) in the 3′-UTR of HIF1A located near the miR-199a binding site was identified [57,94]. The C allele of this variant has an increased frequency in pancreatic ductal adenocarcinoma patients and this CC genotype was characterized by a larger tumour size, shorter overall survival and a higher risk of this disease compared to CT and TT genotypes [57]. Additionally, the occurrence of the C allele was significantly associated with higher HIF1A mRNA and consequently upregulation of HIF1 levels, suggesting that this SNP impairs miR-199a : HIF1A binding [57].
2.3.5. DROSHA | miR-27b
A very interesting example of a synonymous mutation that leads to the loss of an miRNA binding site is SNP rs10719 (T>C) located in the 3′-UTR of the DROSHA gene. The Drosha enzyme, a member of the RNAase III family, plays a critical role in miRNA biogenesis. It liberates the pre-miRNA stem-loop by cleavage of the longer pri-miRNAs in the nucleus [95]. In addition to this function, Drosha also influences cell proliferation and apoptosis [96]. Since overexpression of Drosha is observed in bladder cancer, this SNP is associated with an increased risk of bladder cancer [58]. Yuan et al. [58] reported that DROSHA's 3′-UTR contains a target site for miR-27b, while rs10719 (T>C) is located in close proximity to this site (46 bp downstream of the miR-27b binding site). They have postulated that rs10719T to C transition leads to weaker mRNA : miRNA association at the miR-27b target site and consequently to increased Drosha expression.
2.4. SNPs affecting the miRNA : mRNA interaction
2.4.1. FGF20 | miR-433
An example of another poly-miRTS related to PD was provided by Wang et al. [59], who reported a correlation between SNP (rs127202208 C/T) in the 3′-UTR of fibroblast growth factor 20 (FGF20) and the development of PD. FGF20 is mainly expressed in substantia nigra and increases proliferation and promotes survival of dopaminergic neurons during the early stages of life. However, increased levels of FGF20 in the later stages of life enhance α-synuclein expression and can lead to the death of dopaminergic neurons [59]. miR-433, which is abundant in brain, was shown to downregulate the translation of FGF20, mainly because this reported SNP resides within the predicted binding site for miR-433. The allele C of this polymorphism represents a valid miRNA base pairing, whereas the T allele introduces a G : U wobble base pairing and consequently a mismatch, which affects the miRNA : mRNA interaction. However, this SNP does not eliminate the miRNA : mRNA binding, but attenuates it. This leads to increased FGF20 levels and indirectly to overexpression of α-synuclein. Importantly, the effect of this SNP on FGF20 expression and its relationship to miR-433 levels were confirmed in vivo [59].
3. Conclusion
The discussed examples of poly-miRTSs strongly suggest that these SNPs can be crucial factors in developing human pathologies and could contribute to genetic diversity. As mentioned, roughly 180 000 SNPs in the human genome that are located in the 3'-UTR region were identified along with about 2600 mature miRNA sequences which are deposited in the mirBase (v. 21), suggesting that a large number of these SNPs may introduce miRNA-binding changes. Furthermore, the recent development of deep sequencing techniques and advanced database/software tools like miRSNP and PolymiRTS Database 3.0 (see table 2 for complete list) allows researchers to initially access potential poly-miRTSs. Hence, in the near future, we can expect growing numbers of studies linking poly-miRTSs to human diseases.
Table 2.
Current software and databases dedicated for poly-miRTS studies.
| name | website | applications | refs |
|---|---|---|---|
| polymiRTS Database 3.0 | http://compbio.uthsc.edu/miRSNP/ | SNPs and INDELs in miRNA target sites identified from various experiments, predicted miRNA target sites, miRNA seeds | [97] |
| miRSNP | http://bioinfo.bjmu.edu.cn/mirsnp/search/ | SNPs in predicted miRNA target sites | [98] |
| microRNA-related single nucleotide polymorphism | http://www.bioguo.org/miRNASNP/ | SNPs in human pre-miRNAs, in human miRNA flanks, in miRNAs of other species, target gain/loss by SNP in miRNA seed or in target 3′-UTR | [21] |
| miRdSNP | http://mirdsnp.ccr.buffalo.edu/ | disease-associated SNPs and microRNA target sites on 3′-UTRs of human genes | [99] |
| ImiRP (illegitimate microRNA predictor) | http://imirp.org/ | mutations in predicted miRNAs target sites | [100] |
In 2008, Sethupathy & Collins [17] provided criteria for assigning SNPs as poly-miRTSs involved in human diseases that include: (i) functional (preferably in vivo) experimental validation of SNPs related to differential mRNA targeting; (ii) genetic testing of the association with the disease that takes into account the effects of population stratification; and finally (iii) mechanistic testing underlying the mechanism by which poly-miRTSs contribute to the disease [17]. Few current studies satisfy all these criteria (table 1), while the majority of them rely on population correlation effects and in silico modelling only, ignoring the necessity of the mechanistic approach. Importantly, commonly used methods to confirm differential miRNA : mRNA binding, in vitro luciferase reporter constructs and miRNA overexpression often do not consider the physiological miRNA levels in vivo. However, miRNA physiological levels are often undergoing dynamic changes due to epigenetic factors [101], and thus they can affect the verification of the poly-miRTS disease-related mechanisms. The luciferase-based reporter assays are usually performed in artificial cancer cell lines that permit easy AgoMiR (mimic) delivery, and are often characterized by low endogenous miRNA levels. The latter inhibits endogenous miRNAs from degrading the reporters prior to the miRNA overexpression. Importantly, the miRNA overexpression in these systems is often a hundred fold higher than in vivo conditions. Hence, in the case of validation of new target sites created by poly-miRTS, this experimental model may lead to false positive results, since it cannot differentiate between weak and strong binding to the targets. The vector-based miRNA expression system that provides inducible and scalable control over miRNA levels may provide more solid verification of potential miRNA : mRNA binding [102].
Recently, the development of morpholino-based target protector technology provides an elegant tool to test the functionality of novel potential miRNA : mRNA interactions that mimics physiological conditions [103,104]. Target protectors bind to specific target mRNA sequences and block miRNA access, however without triggering an RNAi response [105]. Hence, target protectors allow blocking the miRNA-mediated suppression of a specific target mRNA [105]. Importantly, these modified oligonucleotides can be used to evaluate the significance of miRNA : mRNA interactions in the context of physiological miRNA levels.
Furthermore, often changes in a gene's mRNA level are not reflected in its protein levels [106]. Hence, the studies of miRNA SNP-affected targets should be always accompanied by monitoring protein levels in cell lines related to the disease. Finally, although in research models usually one miRNA and one target are considered, the single miRNA usually is predicted to bind hundreds of target mRNAs, and have multiple effects on cellular metabolism. Hence, studying the mechanism of poly-miRTS involvement in human diseases requires verification that the miRNA effects are a result of indirect disease-related targets. Although this possibility cannot be totally eliminated, following genome-wide effects of specific miRNA modulation (with next generation sequencing) can support direct miRNA : mRNA interactions.
The most convincing and final criterion for linking poly-miRTSs to disease is establishing the disease-related mechanisms of differential miRNA binding. Taking into account complexity of a potential SNP effect on miRNA : mRNA pairing, this can be challenging. Nevertheless, the recent development of targeted genome editing tools (like CRISPR/Cas9 systems) allows one to make efficient, precise and targeted changes to the genome of the living cells, and opens novel possibilities to overcome this limitation [107]. Sadly, to date, no study has been reported in which targeted genome editing was applied in order to validate poly-miRTSs.
Analysing the specific effects of homozygotic and heterozygotic SNPs in both in vitro and in vivo disease models could provide the critical proof for the role of and frequency that poly-miRTSs occur in human diseases.
Authors' contributions
M.G and A.M. wrote the manuscript, and R.B and J.F.C. revised the paper.
Competing interests
The authors declare no competing financial interests.
Funding
This work has been supported by National Science Center OPUS Program under contract UMO-2015/17/B/NZ3/01485 (to R.B.).
References
- 1.Roses AD. 2000. Pharmacogenetics and the practice of medicine. Nature 405, 857–865. (doi:10.1038/35015728) [DOI] [PubMed] [Google Scholar]
- 2.Levy S, et al. 2007. The diploid genome sequence of an individual human. PLoS Biol. 5, e254 (doi:10.1371/journal.pbio.0050254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu J, Jiang R. 2013. Prediction of deleterious nonsynonymous single-nucleotide polymorphism for human diseases. Sci. World J. 2013, 675851 (doi:10.1155/2013/675851) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savas S, Tuzmen S, Ozcelik H. 2006. Human SNPs resulting in premature stop codons and protein truncation. Hum. Genom. 2, 274–286. (doi:10.1186/1479-7364-2-5-274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shastry BS. 2007. SNPs in disease gene mapping, medicinal drug development and evolution. J. Hum. Genet. 52, 871–880. (doi:10.1007/s10038-007-0200-z) [DOI] [PubMed] [Google Scholar]
- 6.Ng PC, Henikoff S. 2006. Predicting the effects of amino acid substitutions on protein function. Annu. Rev. Genom. Hum. Genet. 7, 61–80. (doi:10.1146/annurev.genom.7.080505.115630) [DOI] [PubMed] [Google Scholar]
- 7.Yue P, Moult J. 2006. Identification and analysis of deleterious human SNPs. J. Mol. Biol. 356, 1263–1274. (doi:10.1016/j.jmb.2005.12.025) [DOI] [PubMed] [Google Scholar]
- 8.Risch NJ. 2000. Searching for genetic determinants in the new millennium. Nature 405, 847–856. (doi:10.1038/35015718) [DOI] [PubMed] [Google Scholar]
- 9.Sauna ZE, Kimchi-Sarfaty C. 2011. Understanding the contribution of synonymous mutations to human disease. Nat. Rev. Genet. 12, 683–691. (doi:10.1038/nrg3051) [DOI] [PubMed] [Google Scholar]
- 10.Sauna ZE, Kimchi-Sarfaty C, Ambudkar SV, Gottesman MM. 2007. Silent polymorphisms speak: how they affect pharmacogenomics and the treatment of cancer. Cancer Res. 67, 9609–9612. (doi:10.1158/0008-5472.CAN-07-2377) [DOI] [PubMed] [Google Scholar]
- 11.Lazrak A, et al. 2013. The silent codon change I507-ATC->ATT contributes to the severity of the DeltaF508CFTR channel dysfunction. FASEB J. 27, 4630–4645. (doi:10.1096/fj.13-227330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartoszewski RA, Jablonsky M, Bartoszewska S, Stevenson L, Dai Q, Kappes J, Collawn JF, Bebok Z. 2010. A synonymous single nucleotide polymorphism in DeltaF508 CFTR alters the secondary structure of the mRNA and the expression of the mutant protein. J. Biol. Chem. 285, 28 741–28 748. (doi:10.1074/jbc.M110.154575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tak YG, Farnham PJ. 2015. Making sense of GWAS: using epigenomics and genome engineering to understand the functional relevance of SNPs in non-coding regions of the human genome. Epigenet. Chromatin 8, 57 (doi:10.1186/s13072-015-0050-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hudson TJ. 2003. Wanted: regulatory SNPs. Nat. Genet. 33, 439–440. (doi:10.1038/ng0403-439) [DOI] [PubMed] [Google Scholar]
- 15.Krawczak M, Reiss J, Cooper DN. 1992. The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: causes and consequences. Hum. Genet. 90, 41–54. (doi:10.1007/BF00210743) [DOI] [PubMed] [Google Scholar]
- 16.Li G, Pan T, Guo D, Li LC. 2014. Regulatory variants and disease: the E-cadherin-160C/A SNP as an example. Mol. Biol. Int. 2014, 967565 (doi:10.1155/2014/967565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sethupathy P, Collins FS. 2008. MicroRNA target site polymorphisms and human disease. Trends Genet. 24, 489–497. (doi:10.1016/j.tig.2008.07.004) [DOI] [PubMed] [Google Scholar]
- 18.Sherry ST, et al. 2001. dbSNP: The NCBI database of genetic variation. Nucleic Acids Res. 29, 308–311. (doi:10.1093/nar/29.1.308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao F, Wigneshweraraj SR, Weinzierl R, Wang Y-P, Buck M. 2009. miRecords: an integrated resource for microRNA–target interactions. Nucleic Acids Res. 37, 4482–4497. (doi:10.1093/nar/gkp419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urbich C, Kuehbacher A, Dimmeler S. 2008. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc. Res. 79, 581–588. (doi:10.1093/cvr/cvn156) [DOI] [PubMed] [Google Scholar]
- 21.Gong J, Tong Y, Zhang H-M, Wang K, Hu T, Shan G, Sun J, Guo A-Y. 2012. Genome-wide identification of SNPs in microRNA genes and the SNP effects on microRNA target binding and biogenesis. Hum. Mutat. 33, 254–263. (doi:10.1002/humu.21641) [DOI] [PubMed] [Google Scholar]
- 22.Gong J, et al. 2015. An update of miRNASNP database for better SNP selection by GWAS data, miRNA expression and online tools. Database 2015, pbav29 (doi:10.1093/database/bav029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai T, et al. 2017. Polymorphisms in MIR499A and MIR125A gene are associated with autoimmune thyroid diseases. Mol. Cell. Endocrinol. 440, 106–115. (doi:10.1016/j.mce.2016.11.017) [DOI] [PubMed] [Google Scholar]
- 24.Mullany LE, Herrick JS, Wolff RK, Slattery ML. 2016. Single nucleotide polymorphisms within microRNAs, microRNA targets, and microRNA biogenesis genes and their impact on colorectal cancer survival. Genes Chromosomes Cancer 56, 285–295. (doi:10.1002/gcc.22434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Han Z, Yang C. In press. Associations of microRNA single nucleotide polymorphisms and disease risk and pathophysiology. Clin. Genet. (doi:10.1111/cge.12950) [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Choi GH, Ko KH, Kim JO, Oh SH, Park YS, Kim OJ, Kim NK. 2016. Association of the single nucleotide polymorphisms in microRNAs 130b, 200b, and 495 with ischemic stroke susceptibility and post-stroke mortality. PLoS ONE 11, e0162519 (doi:10.1371/journal.pone.0162519) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morales S, Gulppi F, Gonzalez-Hormazabal P, Fernandez-Ramires R, Bravo T, Reyes JM, Gomez F, Waugh E, Jara L. 2016. Association of single nucleotide polymorphisms in Pre-miR-27a, Pre-miR-196a2, Pre-miR-423, miR-608 and Pre-miR-618 with breast cancer susceptibility in a South American population. BMC Genet. 17, 109 (doi:10.1186/s12863-016-0415-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartel DP. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297. (doi:10.1016/S0092-8674(04)00045-5) [DOI] [PubMed] [Google Scholar]
- 29.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. 2009. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat. Cell Biol. 11, 228–234. (doi:10.1038/ncb0309-228) [DOI] [PubMed] [Google Scholar]
- 30.Shin C, Nam J-W, Farh KK-H, Chiang HR, Shkumatava A, Bartel DP. 2010. Expanding the microRNA targeting code: functional sites with centered pairing. Mol. Cell 38, 789–802. (doi:10.1016/j.molcel.2010.06.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis BP, Burge CB, Bartel DP. 2005. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120, 15–20. (doi:10.1016/j.cell.2004.12.035) [DOI] [PubMed] [Google Scholar]
- 32.Sood P, Krek A, Zavolan M, Macino G, Rajewsky N. 2006. Cell-type-specific signatures of microRNAs on target mRNA expression. Proc. Natl Acad. Sci. USA 103, 2746–2751. (doi:10.1073/pnas.0511045103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartel DP. 2009. MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233. (doi:10.1016/j.cell.2009.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Filipowicz W, Bhattacharyya SN, Sonenberg N. 2008. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet. 9, 102–114. (doi:10.1038/nrg2290) [DOI] [PubMed] [Google Scholar]
- 35.Esquela-Kerscher A, Slack FJ. 2006. Oncomirs—microRNAs with a role in cancer. Nat. Rev. Cancer 6, 259–269. (doi:10.1038/nrc1840) [DOI] [PubMed] [Google Scholar]
- 36.Agarwal V, Bell GW, Nam JW, Bartel DP. 2015. Predicting effective microRNA target sites in mammalian mRNAs. eLife 4, e05005 (doi:10.7554/eLife.05005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grimson A, Farh KK-H, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. 2007. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol. Cell 27, 91–105. (doi:10.1016/j.molcel.2007.06.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brennecke J, Stark A, Russell RB, Cohen SM. 2005. Principles of microRNA-target recognition. PLoS Biol. 3, e85 (doi:10.1371/journal.pbio.0030085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salzman DW, Weidhaas JB. 2013. SNPing cancer in the bud: microRNA and microRNA-target site polymorphisms as diagnostic and prognostic biomarkers in cancer. Pharmacol. Ther. 137, 55–63. (doi:10.1016/j.pharmthera.2012.08.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jens M, Rajewsky N. 2015. Competition between target sites of regulators shapes post-transcriptional gene regulation. Nat. Rev. Genet. 16, 113–126. (doi:10.1038/nrg3853) [DOI] [PubMed] [Google Scholar]
- 41.Garcia DM, Baek D, Shin C, Bell GW, Grimson A, Bartel DP. 2011. Weak seed-pairing stability and high target-site abundance decrease the proficiency of lsy-6 and other microRNAs. Nat. Struct. Mol. Biol. 18, 1139–1146. (doi:10.1038/nsmb.2115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao F, Xiong X, Pan W, Yang X, Zhou C, Yuan Q, Zhou L, Yan M. 2015. A regulatory MDM4 genetic variant locating in the binding sequence of multiple microRNAs contributes to susceptibility of small cell lung cancer. PLoS ONE 10, e0135647 (doi:10.1371/journal.pone.0135647) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stegeman S, Moya L, Selth LA, Spurdle AB, Clements JA, Batra J. 2015. A genetic variant of MDM4 influences regulation by multiple microRNAs in prostate cancer. Endocr. Relat. Cancer 22, 265–276. (doi:10.1530/ERC-15-0013) [DOI] [PubMed] [Google Scholar]
- 44.Wynendaele J, et al. 2010. An illegitimate microRNA target site within the 3' UTR of MDM4 affects ovarian cancer progression and chemosensitivity. Cancer Res. 70, 9641–9649. (doi:10.1158/0008-5472.CAN-10-0527) [DOI] [PubMed] [Google Scholar]
- 45.Fan C, Wei J, Yuan C, Wang X, Jiang C, Zhou C, Yang M, Krahe R et al. . 2014. The functional TP53 rs1042522 and MDM4 rs4245739 genetic variants contribute to non-Hodgkin lymphoma risk. PLoS ONE 9, e107047 (doi:10.1371/journal.pone.0107047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou L, et al. 2013. Association of a genetic variation in a miR-191 binding site in MDM4 with risk of esophageal squamous cell carcinoma. PLoS ONE 8, e64331 (doi:10.1371/journal.pone.0064331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Y, Gao W, Ding X, Xu W, Liu D, Su B, Sun Y. 2017. Variations within 3'-UTR of MDM4 gene contribute to clinical outcomes of advanced non-small cell lung cancer patients following platinum-based chemotherapy. Oncotarget 8, 16 313–16 324. (doi:10.18632/oncotarget.10771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang M, et al. 2016. A functional variant in TP63 at 3q28 associated with bladder cancer risk by creating an miR-140-5p binding site. Int. J. Cancer 139, 65–74. (doi:10.1002/ijc.29978) [DOI] [PubMed] [Google Scholar]
- 49.Goda N, Murase H, Kasezawa N, Goda T, Yamakawa-Kobayashi K. 2015. Polymorphism in microRNA-binding site in HNF1B influences the susceptibility of type 2 diabetes mellitus: a population based case-control study. BMC Med. Genet. 16, 75 (doi:10.1186/s12881-015-0219-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu SL, Cui GL, Huang J, Jiang JG, Wang DW. 2016. An APOC3 3'UTR variant associated with plasma triglycerides levels and coronary heart disease by creating a functional miR-4271 binding site. Sci. Rep. 6, 32700 (doi:10.1038/srep32700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caussy C, et al. 2014. An APOA5 3' UTR variant associated with plasma triglycerides triggers APOA5 downregulation by creating a functional miR-485-5p binding site. Am. J. Hum. Genet. 94, 129–134. (doi:10.1016/j.ajhg.2013.12.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richardson K, et al. 2011. The PLIN4 variant rs8887 modulates obesity related phenotypes in humans through creation of a novel miR-522 seed site. PLoS ONE 6, e17944 (doi:10.1371/journal.pone.0017944) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bandiera S, et al. 2013. Genetic variations creating microRNA target sites in the FXN 3'-UTR affect frataxin expression in Friedreich ataxia. PLoS ONE 8, e54791 (doi:10.1371/journal.pone.0054791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kabaria S, Choi DC, Chaudhuri AD, Mouradian MM, Junn E. 2015. Inhibition of miR-34b and miR-34c enhances alpha-synuclein expression in Parkinson's disease. FEBS Lett. 589, 319–325. (doi:10.1016/j.febslet.2014.12.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gilam A, Conde J, Weissglas-Volkov D, Oliva N, Friedman E, Artzi N, Shomron N. 2016. Local microRNA delivery targets Palladin and prevents metastatic breast cancer. Nat. Commun. 7, 12868 (doi:10.1038/ncomms12868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu S, et al. 2016. MicroRNA-137 inhibits EFNB2 expression affected by a genetic variant and is expressed aberrantly in peripheral blood of schizophrenia patients. EBioMedicine 12, 133–142. (doi:10.1016/j.ebiom.2016.09.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, et al. 2016. Single nucleotide polymorphism in the microRNA-199a binding site of HIF1A gene is associated with pancreatic ductal adenocarcinoma risk and worse clinical outcomes. Oncotarget 7, 13 717–13 729. (doi:10.18632/oncotarget.7263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan L, et al. 2013. Genetic variation in DROSHA 3'UTR regulated by hsa-miR-27b is associated with bladder cancer risk. PLoS ONE 8, e81524 (doi:10.1371/journal.pone.0081524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang G, van der Walt JM, Mayhew G, Li Y-J, Züchner S, Scott WK, Martin ER, Vance JM. 2008. Variation in the miRNA-433 binding site of FGF20 confers risk for Parkinson disease by overexpression of alpha-synuclein. Am. J. Hum. Genet. 82, 283–289. (doi:10.1016/j.ajhg.2007.09.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abelson JF, et al. 2005. Sequence variants in SLITRK1 are associated with Tourette's syndrome. Science 310, 317–320. (doi:10.1126/science.1116502) [DOI] [PubMed] [Google Scholar]
- 61.Zuchner S, Wang G, Tran-Viet K-N, Nance MA, Gaskell PC, Vance JM, Ashley-Koch AE, Pericak-Vance MA. 2006. Mutations in the novel mitochondrial protein REEP1 cause hereditary spastic paraplegia type 31. Am. J. Hum. Genet. 79, 365–369. (doi:10.1086/505361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beetz C, et al. 2008. REEP1 mutation spectrum and genotype/phenotype correlation in hereditary spastic paraplegia type 31. Brain 131, 1078–1086. (doi:10.1093/brain/awn026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adams BD, Furneaux H, White BA. 2007. The micro-ribonucleic acid (miRNA) miR-206 targets the human estrogen receptor-alpha (ERalpha) and represses ERalpha messenger RNA and protein expression in breast cancer cell lines. Mol. Endocrinol. 21, 1132–1147. (doi:10.1210/me.2007-0022) [DOI] [PubMed] [Google Scholar]
- 64.Sethupathy P, Borel C, Gagnebin M, Grant GR, Deutsch S, Elton TS, Hatzigeorgiou AG, Antonarakis SE. 2007. Human microRNA-155 on chromosome 21 differentially interacts with its polymorphic target in the AGTR1 3′ untranslated region: a mechanism for functional single-nucleotide polymorphisms related to phenotypes. Am. J. Hum. Genet. 81, 405–413. (doi:10.1086/519979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mishra PJ, Humeniuk R, Longo-Sorbello GS, Banerjee D, Bertino JR. 2007. A miR-24 microRNA binding-site polymorphism in dihydrofolate reductase gene leads to methotrexate resistance. Proc. Natl Acad. Sci. USA 104, 13 513–13 518. (doi:10.1073/pnas.0706217104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan Z, et al. 2007. Allele-specific targeting of microRNAs to HLA-G and risk of asthma. Am. J. Hum. Genet. 81, 829–834. (doi:10.1086/521200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jensen KP, Covault J, Conner TS, Tennen H, Kranzler HR, Furneaux HM. 2009. A common polymorphism in serotonin receptor 1B mRNA moderates regulation by miR-96 and associates with aggressive human behaviors. Mol. Psychiatry 14, 381–389. (doi:10.1038/mp.2008.15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Landi D, et al. 2008. Polymorphisms within micro-RNA-binding sites and risk of sporadic colorectal cancer. Carcinogenesis 29, 579–584. (doi:10.1093/carcin/bgm304) [DOI] [PubMed] [Google Scholar]
- 69.Kapeller J, et al. 2008. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type 3E gene with diarrhea predominant irritable bowel syndrome. Hum. Mol. Genet. 17, 2967–2977. (doi:10.1093/hmg/ddn195) [DOI] [PubMed] [Google Scholar]
- 70.Wade M, Wang YV, Wahl GM. 2010. The p53 orchestra: Mdm2 and Mdmx set the tone. Trends Cell Biol. 20, 299–309. (doi:10.1016/j.tcb.2010.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu C, et al. 2016. MDM4 rs4245739 A > C polymorphism correlates with reduced overall cancer risk in a meta-analysis of 69477 subjects. Oncotarget 7, 71 718–71 726. (doi:10.18632/oncotarget.12326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Westfall MD, Pietenpol JA. 2004. p63: Molecular complexity in development and cancer. Carcinogenesis 25, 857–864. (doi:10.1093/carcin/bgh148) [DOI] [PubMed] [Google Scholar]
- 73.Park E, Kim H, Kim JM, Primack B, Vidal-Cardenas S, Xu Y, Price BD, Mills AA, D'Andrea AD. 2013. FANCD2 activates transcription of TAp63 and suppresses tumorigenesis. Mol. Cell 50, 908–918. (doi:10.1016/j.molcel.2013.05.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo X, Keyes WM, Papazoglu C, Zuber J, Li W, Lowe SW, Vogel H, Mills AA. 2009. TAp63 induces senescence and suppresses tumorigenesis in vivo. Nat. Cell Biol. 11, 1451–1457. (doi:10.1038/ncb1988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Flores ER, Tsai KY, Crowley D, Sengupta S, Yang A, McKeon F, Jacks T. 2002. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature 416, 560–564. (doi:10.1038/416560a) [DOI] [PubMed] [Google Scholar]
- 76.Coffinier C, Thepot D, Babinet C, Yaniv M, Barra J. 1999. Essential role for the homeoprotein vHNF1/HNF1 beta in visceral endoderm differentiation. Development 126, 4785–4794. [DOI] [PubMed] [Google Scholar]
- 77.Kornfeld JW, et al. 2013. Obesity-induced overexpression of miR-802 impairs glucose metabolism through silencing of Hnf1b. Nature 494, 111–115. (doi:10.1038/nature11793) [DOI] [PubMed] [Google Scholar]
- 78.Marinescu VD, Kohane IS, Riva A. 2005. MAPPER: a search engine for the computational identification of putative transcription factor binding sites in multiple genomes. BMC Bioinformatics 6, 79 (doi:10.1186/1471-2105-6-79) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andersson I, Arner P, Mottagui-Tabar S, Rydén M, Löfgren P, Faulds G, Hoffstedt J, Brookes AJ. 2003. Evidence for an important role of perilipin in the regulation of human adipocyte lipolysis. Diabetologia 46, 789–797. (doi:10.1007/s00125-003-1112-x) [DOI] [PubMed] [Google Scholar]
- 80.Wang Y, et al. 2003. Perilipin expression in human adipose tissues: effects of severe obesity, gender, and depot. Obes. Res. 11, 930–936. (doi:10.1038/oby.2003.128) [DOI] [PubMed] [Google Scholar]
- 81.Marmolino D. 2011. Friedreich's ataxia: past, present and future. Brain Res. Rev. 67, 311–330. (doi:10.1016/j.brainresrev.2011.04.001) [DOI] [PubMed] [Google Scholar]
- 82.Sonntag KC, Woo TU, Krichevsky AM. 2012. Converging miRNA functions in diverse brain disorders: a case for miR-124 and miR-126. Exp. Neurol. 235, 427–435. (doi:10.1016/j.expneurol.2011.11.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mahishi LH, Hart RP, Lynch DR, Ratan RR. 2012. miR-886-3p levels are elevated in Friedreich ataxia. J. Neurosci. 32, 9369–9373. (doi:10.1523/JNEUROSCI.0059-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sotiriou S, Gibney G, Baxevanis AD, Nussbaum RL. 2009. A single nucleotide polymorphism in the 3'UTR of the SNCA gene encoding alpha-synuclein is a new potential susceptibility locus for Parkinson disease. Neurosci. Lett. 461, 196–201. (doi:10.1016/j.neulet.2009.06.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Del Tredici K, Braak H. 2016. Review: Sporadic Parkinson's disease: development and distribution of alpha-synuclein pathology. Neuropathol. Appl. Neurobiol. 42, 33–50. (doi:10.1111/nan.12298) [DOI] [PubMed] [Google Scholar]
- 86.Rehmsmeier M, Steffen P, Hochsmann M, Giegerich R. 2004. Fast and effective prediction of microRNA/target duplexes. RNA 10, 1507–1517. (doi:10.1261/rna.5248604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goicoechea SM, Bednarski B, García-Mata R, Prentice-Dunn H, Kim HJ, Otey CA. 2009. Palladin contributes to invasive motility in human breast cancer cells. Oncogene 28, 587–598. (doi:10.1038/onc.2008.408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gucciardo E, Lohi J, Li R, Sugiyama N, Carpen O, Lehti K. 2014. Actin-associated protein palladin promotes tumor cell invasion by linking extracellular matrix degradation to cell cytoskeleton. Mol. Biol. Cell 25, 2556–2570. (doi:10.1091/mbc.E13-11-0667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Senturk A, Pfennig S, Weiss A, Burk K, Acker-Palmer A. 2011. Ephrin Bs are essential components of the Reelin pathway to regulate neuronal migration. Nature 472, 356–360. (doi:10.1038/nature09874) [DOI] [PubMed] [Google Scholar]
- 90.Janaszak-Jasiecka A, Bartoszewska S, Kochan K, Piotrowski A, Kalinowski L, Kamysz W, Ochocka RJ. 2016. miR-429 regulates the transition between hypoxia-inducible factor (HIF)1A and HIF3A expression in human endothelial cells. Sci. Rep. 6, 22775 (doi:10.1038/srep22775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bartoszewska S, Kochan K, Piotrowski A, Kamysz W, Ochocka RJ, Collawn JF, Bartoszewski R. 2015. The hypoxia-inducible miR-429 regulates hypoxia-inducible factor-1α expression in human endothelial cells through a negative feedback loop. FASEB J 29, 1467–1479. (doi:10.1096/fj.14-267054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Madanecki P, Kapoor N, Bebok Z, Ochocka R, Collawn J, Bartoszewski R. 2013. Regulation of angiogenesis by hypoxia: the role of microRNA. Cell Mol. Biol. Lett. 18, 47–57. (doi:10.2478/s11658-012-0037-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rane S, He M, Sayed D, Vashistha H, Malhotra A, Sadoshima J, Vatner DE, Vatner SF, Abdellatif M. 2009. Downregulation of MiR-199a derepresses hypoxia-inducible factor-1α and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ. Res. 104, 879–886. (doi:10.1161/CIRCRESAHA.108.193102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guo X, Li D, Chen Y, An J, Wang K, Xu Z, Chen Z, Xing J. 2015. SNP rs2057482 in HIF1A gene predicts clinical outcome of aggressive hepatocellular carcinoma patients after surgery. Sci. Rep. 5, 11846 (doi:10.1038/srep11846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gurtner A, Falcone E, Garibaldi F, Piaggio G. 2016. Dysregulation of microRNA biogenesis in cancer: the impact of mutant p53 on Drosha complex activity. J. Exp. Clin. Cancer Res. 35, 45 (doi:10.1186/s13046-016-0319-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Z, Zhang G, Kong C, Bi J, Gong D, Yu X, Shi D, Zhan B, Ye P. 2015. EIF2C, Dicer, and Drosha are up-regulated along tumor progression and associated with poor prognosis in bladder carcinoma. Tumour Biol. 36, 5071–5079. (doi:10.1007/s13277-015-3158-z) [DOI] [PubMed] [Google Scholar]
- 97.Bhattacharya A, Ziebarth JD, Cui Y. 2014. PolymiRTS Database 3.0: linking polymorphisms in microRNAs and their target sites with human diseases and biological pathways. Nucleic Acids Res. 42, D86–D91. (doi:10.1093/nar/gkt1028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu C, Zhang F, Li T, Lu M, Wang L, Yue W, Zhang D. 2012. MirSNP, a database of polymorphisms altering miRNA target sites, identifies miRNA-related SNPs in GWAS SNPs and eQTLs. BMC Genomics 13, 661 (doi:10.1186/1471-2164-13-661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bruno AE, Li L, Kalabus JL, Pan Y, Yu A, Hu Z. 2012. miRdSNP: a database of disease-associated SNPs and microRNA target sites on 3'UTRs of human genes. BMC Genomics 13, 44 (doi:10.1186/1471-2164-13-44) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ryan BC, Werner TS, Howard PL, Chow RL. 2016. ImiRP: a computational approach to microRNA target site mutation. BMC Bioinform. 17, 190 (doi:10.1186/s12859-016-1057-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Melo SA, Esteller M. 2011. Dysregulation of microRNAs in cancer: playing with fire. FEBS Lett. 585, 2087–2099. (doi:10.1016/j.febslet.2010.08.009) [DOI] [PubMed] [Google Scholar]
- 102.Shin K-J, et al. 2006. A single lentiviral vector platform for microRNA-based conditional RNA interference and coordinated transgene expression. Proc. Natl Acad. Sci. USA 103, 13 759–13 764. (doi:10.1073/pnas.0606179103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Amantana A, Iversen PL. 2005. Pharmacokinetics and biodistribution of phosphorodiamidate morpholino antisense oligomers. Curr. Opin. Pharmacol. 5, 550–555. (doi:10.1016/j.coph.2005.07.001) [DOI] [PubMed] [Google Scholar]
- 104.Summerton J. 1999. Morpholino antisense oligomers: the case for an RNase H-independent structural type. Biochim. Biophys. Acta 1489, 141–158. (doi:10.1016/S0167-4781(99)00150-5) [DOI] [PubMed] [Google Scholar]
- 105.Staton AA, Giraldez AJ. 2011. Use of target protector morpholinos to analyze the physiological roles of specific miRNA-mRNA pairs in vivo. Nat. Protoc. 6, 2035–2049. (doi:10.1038/nprot.2011.423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vogel C, Marcotte EM. 2012. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 13, 227–232. (doi:10.1038/nrg3185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Porteus MH. 2015. Towards a new era in medicine: therapeutic genome editing. Genome Biol. 16, 286 (doi:10.1186/s13059-015-0859-y) [DOI] [PMC free article] [PubMed] [Google Scholar]