Abstract
The success of early life-history stages is an environmentally sensitive bottleneck for many marine invertebrates. Responses of larvae to environmental stress may vary due to differences in maternal investment of energy stores and acclimatization/adaptation of a population to local environmental conditions. In this study, we compared two populations from sites with different environmental regimes (Moorea and Taiwan). We assessed the responses of Pocillopora damicornis larvae to two future co-occurring environmental stressors: elevated temperature and ocean acidification. Larvae from Taiwan were more sensitive to temperature, producing fewer energy-storage lipids under high temperature. In general, planulae in Moorea and Taiwan responded similarly to pCO2. Additionally, corals in the study sites with different environments produced larvae with different initial traits, which may have shaped the different physiological responses observed. Notably, under ambient conditions, planulae in Taiwan increased their stores of wax ester and triacylglycerol in general over the first 24 h of their dispersal, whereas planulae from Moorea consumed energy-storage lipids in all cases. Comparisons of physiological responses of P. damicornis larvae to conditions of ocean acidification and warming between sites across the species' biogeographic range illuminates the variety of physiological responses maintained within P. damicornis, which may enhance the overall persistence of this species in the light of global climate change.
Keywords: coral, lipid, environmental history, ocean acidification, ocean warming, larvae
1. Introduction
Early life-history stages of corals are increasingly threatened by global anthropogenic stressors, such as ocean acidification (OA), the decrease in pH and carbonate ion concentration caused by the absorption of anthropogenic CO2 by the surface ocean, and ocean warming. For coral larvae, many biological traits and processes vary with pCO2 conditions, with exceptions [1–7]. The biological responses of coral larvae to ocean acidification and concurrent warming will likely affect their dispersal through the depletion of energy stores and a reduction in larval buoyancy [8–10]. Being lecithotrophic, most coral larvae use lipids and protein as endogenous sources of energy throughout dispersal [8,10,11]. Translocated metabolites from Symbiodinium are also important sources of energy for dispersing larvae [12,13]. Temperature stress can decouple the physiology of symbiosis in coral larvae (Porites astreoides, [14]), and the thermotolerance ceiling could be worsened by the addition of OA. Additionally, in response to OA and warming, upregulation of stress response pathways and increased maintenance of homeostatic processes may necessitate increased consumption of energy stores (i.e. lipid and protein) or shifts in the allocation of metabolic energy. Trade-offs of these responses to environmental stress include slowed developmental timing, reduced growth and impaired competency to settle (e.g. [14–16]). Furthermore, reduction of lipid stores will decrease larval buoyancy, altering the physical transport of larvae by currents [17]. As a consequence of reduced energy stores and buoyancy, larvae may disperse shorter distances or may terminate in the plankton, with negative consequences for suitable habitat selection, recruitment success, restoration of damaged populations and region-wide connectivity.
At the population level, the effects of OA and warming may be mediated by local environmental conditions. Adaptation to local regimes of pH and temperature occurs over a sufficient spatial scale where environmental conditions differ consistently and where gene flow is low [18–20], and in this context, local adaptation to temperature has been well studied [21,22]. Studies in other systems provide evidence that local adaptation to pH regimes can occur in nature [23–25]. If coral populations are locally adapted to their environmental regime, corals living in a naturally more acidic environment may have developed physiological tools for maintaining normal function in low-pH seawater, as has been shown for elevated temperature [26]. The role of environmental history in shaping the response of coral populations to combined effects of OA and warming remains unknown.
In this study, we assessed the ecological development (‘the mechanisms of developmental regulation in real-world environments’, [27]) of P. damicornis larvae from populations with different environmental histories. Specifically, we examined the effects of OA and temperature on larval performance. To do this, we performed CO2 and temperature manipulation experiments with P. damicornis larvae collected in Moorea, French Polynesia and in Taiwan. These sites were chosen based on our experience working at these locations and a priori knowledge of differing oceanographic regimes, the former being a fringing reef in a sheltered lagoon and the latter experiencing tidally driven upwelling events [28]. We used biochemical composition as an index of performance. Lipid composition and biological traits of larvae were measured both upon release and after exposure to experimental conditions in order to assess how OA and warming might alter larval performance and dispersal. Environmental data using autonomous pH and temperature sensors deployed in both locations provided context for experimental conditions. For this study, we asked: (i) do the effects of OA and warming on lipid composition and other physiological traits of P. damicornis larvae differ between cohorts released at separate biogeographic locations and (ii) do differences in the effects of OA and warming correspond with the environmental history of each coral population? We hypothesized that responses of coral larvae to conditions of OA and warming would differ between locations and that the responses to pCO2 and temperature would correspond with environmental history of these parameters.
2. Material and methods
(a). Collection of coral larvae
During the austral summer in 2012, eight colonies of P. damicornis (electronic supplementary material, figure S1) were collected from fringing reef sites in Moorea, French Polynesia and Taiwan, respectively, at approximately 1–3 m depth (17.4803 S, 149.7989 W; 21.9385 N 120.7967 E). At the Richard B. Gump South Pacific Research Station and at the National Museum of Marine Biology and Aquarium (NMMBA) in Taiwan, larvae were collected and pooled following [29] the lunar pattern of reproduction of P. damicornis [30]. The ‘peak’ larvae, determined by the predicted release curve of the colonies (28 February 2012 in Moorea and 25 June 2012 in Taiwan; e.g. [6,31,32]), were used in the experiment. In Moorea, all larvae released were used in the experiments, resulting in unequal genotype ratios. In Taiwan, equal numbers of larvae from each colony were contributed to this pool. The condition of the freshly released larvae was assessed using lipid metrics and other physiological parameters (see below). The remaining larvae in each pool were then randomly assigned to experimental treatments.
(b). Experimental incubations
Larvae were divided among eight tanks containing four treatment combinations of pCO2 and temperature. Larvae were incubated for 24 h under experimental conditions in two 400 ml containers per aquarium at approximately 0.15–0.25 larva ml−1. These containers had 100 µm mesh sides and a photosynthetically active radiation (PAR)-transparent lid and were anchored in place within the aquarium to ensure that PAR exposure was replicated across tanks. Owing to the time needed to photograph and preserve larvae post-incubation, incubations were staggered by 1 h per aquarium, with the order randomized. At the end of each incubation, larvae within tanks were pooled, and 10 larvae were randomly selected for size measurements (n = 20 per treatment). The remaining larvae were frozen at −80°C in aliquots for downstream analyses of lipid classes (3 × 25 larvae), total protein content (2 × 5 larvae) and symbiont density (2 × 5 larvae). Therefore, for comparisons between treatments, n = 6 for lipid classes, n = 4 for total protein and n = 4 for symbiont density.
In laboratories at both sites, two pCO2 and two temperature treatments were prescribed: ambient temperature–ambient pCO2 (ATAC), ambient temperature–high pCO2 (ATHC), high temperature–ambient pCO2 (HTAC) and high temperature–high pCO2 (HTHC). The ambient CO2 treatment (approx. 400–450 µatm pCO2) approximated current environmental conditions of the water mass bathing the fringing reef where the adult corals were collected (confirmed by environmental data; [32]). The control temperatures (27.5–28°C) approximated the multi-year average temperature for the fringing reefs close to the collection sites for adult P. damicornis ([33], T.-Y. Fan January 2007 to March 2009, March 2010 to present, unpublished data). The high CO2 and high temperature treatments (approx. 900–1000 µatm pCO2, 30.5–31°C) represent ocean conditions expected by the year 2100 under a business-as-usual scenario [34]. See the electronic supplementary material for detailed information on experimental culturing conditions.
To verify and monitor the physical parameters of the OA × temperature treatments, the chemistry of the seawater in the aquaria was analysed during the experiment. pH, temperature, salinity and total alkalinity of seawater in each aquarium were measured during the incubations following best practices (see the electronic supplementary material).
(c). Assessment of cellular lipids
Lipids were extracted from larval homogenates following [35,36] (see the electronic supplementary material for additional details). Total lipid values were obtained from lipid extracts evaporated at 37°C under nitrogen gas. Lipid classes of wax ester (WE), triacylglycerol (TG) and phospholipid (PL) were quantified using two chromatography-based techniques. For larvae sampled in Moorea, a thin-layer chromatography-flame ionization detection analyser was used (Iatroscan MK-5, Iatron Laboratories, Inc., Tokyo, Japan) following [29]. For larvae sampled in Taiwan, these lipid classes were quantified using thin-layer chromatography (TLC; see the electronic supplementary material). Phospholipids were quantified using a spectrophotometric assay for phosphorus determination (modified from [37], see the electronic supplementary material).
(d). Characterization of other larval physiological parameters
To assess other aspects of physiology of incubated larvae, total protein, density of Symbiodinium and larval size were quantified. The Bradford assay was used to quantify total protein ([38,39], following [6]). Symbiodinium density was quantified using a haemocytometer (see the electronic supplementary material). Larval circumference (area) and maximum length were determined from photographs of larvae [40]. For comparisons to other publications, larval volume was calculated following [41] and is included in electronic supplementary material, table S1.
(e). Statistical analyses of biological data
All data were analysed using R version 3.0.1 (R Core Team 2013). Statistical assumptions of normality and homogeneity of variance were met based on Q-Q plots and Levene's test, sometimes following an inverse transformation. Physical experimental conditions were compared using type III sum of squares, with pCO2 and temperature treatments as fixed factors and tank as a random factor. All pre- and post-incubation quantities of lipid classes were standardized to total lipid. For initial conditions of larvae, lipid and other physiological metrics were analysed for effect of Site using a one-way ANOVA. Because initial biological metrics of larvae often differed by site, we compared the change in these metrics over the first 24 h of larval duration between pCO2 exposures, temperature exposures, and sites. To do so, mean pre-incubation levels were subtracted from post-incubation levels, henceforth referred to as Δ, ‘delta’. Therefore, a negative ΔWE per larva represents a net consumption of WE during the 24-h incubation. Absolute values of lipids and other parameters are provided in the electronic supplementary material, tables S1 and S2.
Linear mixed-effect models (nlme package in R; [42]) were used to estimate effects on response variables, with pCO2, temperature and site as fixed factors and aquarium (tank) as a random factor. Model selection was performed incrementally, following [43]. Likelihood ratio tests (type III sum of squares) were conducted on selected models fit using maximum likelihood in order to compare the effects of fixed factors [44,45]. Post-hoc analyses were performed using orthogonal contrasts (multcomp package in R; [46]). General linear hypothesis tests with Bonferroni corrections (GLHT) were used for multiple comparisons, and Tukey's HSD was used for single comparisons.
(f). Environmental data collection
pH and temperature time series were generated on fringing reefs in Moorea, French Polynesia and in Taiwan, within 33 m from the collecting locations of adult P. damicornis parents (see the electronic supplementary material). A full comparison of environmental regimes between sites can be found in [32].
3. Results
At two sites, we measured the effects of OA and warming on lipid composition and other physiological traits of P. damicornis larvae, following a 24-h experimental exposure under laboratory conditions where temperature and seawater chemistry were controlled (table 1). Treatment levels were statistically distinct at each site (see the electronic supplementary material, table S3). Although some incidences of larval death were observed during the incubations, these events were aquarium-specific and were not consistent across treatments or locations.
Table 1.
Summary of physical conditions in experimental aquaria used in Moorea, French Polynesia and in Taiwan. Data are presented as mean ± s.e. For all parameters, n = 6.
| site | treatment | temperature (°C) | salinity (psu) | pHtotal | AT (μmol kg−1) | pCO2 (μatm) |
|---|---|---|---|---|---|---|
| Moorea | ATAC | 27.99 ± 0.10 | 35.82 ± 0.02 | 8.004 ± 0.003 | 2362 ± 1 | 459 ± 4 |
| HTAC | 30.57 ± 0.11 | 35.85 ± 0.02 | 7.970 ± 0.007 | 2363 ± 1 | 496 ± 9 | |
| ATHC | 28.12 ± 0.18 | 35.83 ± 0.02 | 7.719 ± 0.008 | 2364 ± 1 | 1002 ± 22 | |
| HTHC | 30.77 ± 0.20 | 35.88 ± 0.02 | 7.692 ± 0.012 | 2368 ± 2 | 1068 ± 33 | |
| Taiwan | ATAC | 27.75 ± 0.18 | 30.61 ± 0.18 | 7.971 ± 0.007 | 2234 ± 4 | 494 ± 9 |
| HTAC | 30.53 ± 0.05 | 30.63 ± 0.23 | 7.978 ± 0.005 | 2253 ± 6 | 487 ± 9 | |
| ATHC | 27.70 ± 0.20 | 30.26 ± 0.29 | 7.733 ± 0.006 | 2204 ± 15 | 923 ± 14 | |
| HTHC | 30.43 ± 0.04 | 30.93 ± 0.14 | 7.748 ± 0.004 | 2243 ± 6 | 900 ± 9 |
(a). Lipid composition of Pocillopora damicornis larvae
Larvae in Moorea contained on average 32.00 µg total lipid per larva, including 58% WE, 11% TG, and 17% PL, and a mean of 11.65 µg total protein per larva. Larvae in Taiwan contained on average 20.47 µg total lipid per larva, including 39% WE, 18% TG and 6% PL, but a larger total protein fraction, a mean of 22.37 µg per larva.
In P. damicornis larvae immediately after release, initial levels of WE and PL, as a proportion of total lipid, did not vary significantly with Site (see the electronic supplementary material, table S4). However, initial TG varied significantly by Site (p < 0.001; electronic supplementary material, table S4). Larvae in Moorea had significantly less TG than those in Taiwan (Tukey's HSD, electronic supplementary material, table S4 and figure S2). Total lipid varied significantly by Site (p = 0.009; electronic supplementary material, table S4); levels in Moorea were greater than those in Taiwan (Tukey's HSD, electronic supplementary material, table S4 and figure S2).
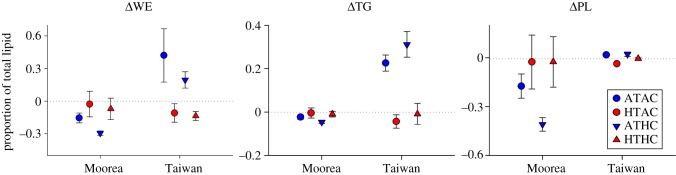
In response to conditions of pCO2 and temperature, ΔWE ranged from −11.47 µg per larva (MCR HTHC; 62% depletion of initial levels) to + 17.64 µg per larva (TWN ATAC; more than 100% increase from initial levels). When normalized by total lipid, ΔWE varied significantly only by T × Site and Site (p < 0.001 for each; see the electronic supplementary material, table S5). Larval WE content increased significantly at Ambient-T for larvae in Taiwan, but not in Moorea (Tukey's HSD, see the electronic supplementary material, table S6; figure 1).
Figure 1.
Changes in lipid composition of P. damicornis larvae over 24-h exposures to combinations of pCO2 and temperature. Mean ± s.e. (n = 6) changes in abundance of WE, TG and PL classes for larvae released in Moorea, French Polynesia and Taiwan. Negative Δ values indicate net decreases in per cent lipid composition over the experimental exposure. Lipid quantities are standardized by total lipid. Experimental treatments: Ambient-T, Ambient-pCO2 (ATAC), High-T, Ambient-pCO2 (HTAC), Ambient-T, High-pCO2 (ATHC), High-T, High-pCO2 (HTHC).
ΔTG varied between −2.38 µg per larva (TWN HTHC; 42% depletion of initial levels) and +7.57 µg per larva (TWN ATHC; more than 100% increase from initial levels). Only the effect of Site was significant for ΔTG, which was standardized by total lipid (p = 0.005; electronic supplementary material, table S5). TG became a larger portion of total lipid for larvae in Taiwan over larvae in Moorea (Tukey's HSD, electronic supplementary material, table S6), particularly noticeable under Ambient-T (figure 1).
ΔPL ranged from −4.00 µg per larva (MCR HTAC; 75% depletion) to +2.99 µg per larva (MCR HTAC; 56% increase). Normalized to total lipid, ΔPL varied significantly by T × Site, T and Site (p = 0.010, p = 0.002, p < 0.001; respectively; electronic supplementary material, table S5). Larvae in Moorea experienced greater depletion of PL at Ambient-T than at High-T, while in Taiwan, ΔPL was similar between temperatures (GLHT, electronic supplementary material, table S6; figure 1).
ΔTL varied between −18.22 µg per larva (MCR HTAC; 57% depletion) and +21.65 µg per larva (MCR ATHC; 68% increase); pCO2 × T × Site and pCO2 × T were significant effects (p = 0.013, p = 0.041, respectively; see the electronic supplementary material, table S7).
(b). Other larval physiological parameters
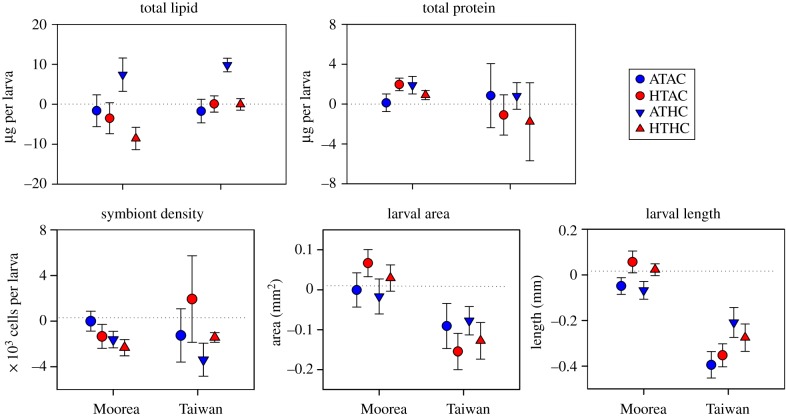
In P. damicornis larvae immediately after release, average Symbiodinium density was 7850 cells per larva in Moorea and 9008 cells per larva in Taiwan. Neither total protein (TP) nor symbiont density differed by Site (electronic supplementary material, table S4). Larval area and length varied significantly by Site (p < 0.001 for each; electronic supplementary material, table S4), with larger larvae released in Taiwan (Tukey's HSD, electronic supplementary material, table S4 and figure S2).
Changes in response to different pCO2 and temperature levels were measured in the form of TP, symbiont density and larval size. ΔTP ranged from −7.43 µg per larva (TWN HTHC; 56% depletion) to +7.53 µg per larva (TWN ATAC; 34% increase). Total protein content of larvae did not vary significantly by pCO2, T or Site (electronic supplementary material, table S7). Changes in symbiont abundance ranged from −6614 cells per larva (TWN ATAC; 73% decrease) to +8558 cells per larva (TWN HTAC; 95% increase). However, symbiont density did not vary significantly by any of the effects tested (electronic supplementary material, table S7). Changes in larval area varied between 69% growth (TWN ATAC) and 64% decrease (TWN HTAC). Only the effect of Site explained a significant amount of variation in this parameter (p < 0.001 electronic supplementary material, table S7), with significantly more growth in larval area in Moorea than in Taiwan (Tukey's HSD, electronic supplementary material, table S8; figure 2). Changes in larval length ranged from −864 µm (TWN ATAC) to +785 µm (MCR ATHC); significant effects included pCO2 and Site (p = 0.043, p = 0.038, respectively; electronic supplementary material, table S7). Larvae became significantly shorter in length in Taiwan versus Moorea (Tukey's HSD, electronic supplementary material, table S8; figure 2) and at Ambient-pCO2 versus High-pCO2 (Tukey's HSD, electronic supplementary material, table S8; figure 2).
Figure 2.
Changes in larval physiological condition for P. damicornis larvae following 24-h exposures to pCO2 and temperature. Mean ± s.e. (n = 6) changes in larval composition and size for larvae released in Moorea, French Polynesia and Taiwan. Negative Δ values indicate net consumption over the experimental exposure. Physiological condition traits are standardized per larva (µg per larva), unless otherwise noted. Experimental treatments: Ambient-T, Ambient-pCO2 (ATAC), High-T, Ambient-pCO2 (HTAC), Ambient-T, High-pCO2 (ATHC), High-T, High-pCO2 (HTHC).
4. Discussion
(a). Variation in maternal investment of Pocillopora damicornis larvae
Measurements of biochemical and biological traits of newly released P. damicornis larvae provide critical contextual information about the physiological condition of freshly released larvae and inform the link between maternal investment and larval performance. P. damicornis larvae in Moorea received more total lipid, though energy-rich lipid classes were equal to or less dense than for larvae released in Taiwan. Based on these initial conditions, particularly for WE, larvae released at both locations contained similar long-term energy stores, which may influence buoyancy and dispersal potential. Additionally, the proportion of total lipid concentrated in long-term storage (WE) versus short-term storage (TG) indicates that in order to mount a physiological response to environmental changes, such as OA, larvae from Moorea will likely need to mobilize part of their WE pool, sacrificing their buoyancy in the process. Larvae from corals of the Taiwan population contained more TG upon release. Maternal investment may prime these larvae for more immediate response to rapidly changing environmental conditions [29]. If utilization of long-term lipid stores is required as well, their dispersal distance may be diminished. In Moorea and elsewhere, brooded P. damicornis larvae commonly disperse several kilometres away from the natal reef, and these populations have genetic similarity [47,48]. However, local retention is also frequently observed [48], and larvae of this species likely do not successfully recruit to other islands in French Polynesia [47].
With equal symbiont densities upon release, larvae of the two populations had similar potential to produce new energy, in the form of fixed carbon, assuming they contained the same clade of Symbiodinium with similar photophysiology. However, the clades of Symbiodinium may have differed between sites, likely appropriate for the local environmental conditions of each location [49–51].
Larval size correlated with total protein and was greater in larvae from Taiwan. Large P. damicornis larvae can survive for a longer period of time in the plankton than small larvae and can therefore potentially disperse farther [41]; however, in this case, it is unknown whether planula size was correlated with lipid or protein content. A longer dispersal period may allow planulae to discover preferred or novel habitats for settlement, but at the cost of extended predation risk and removal from favourable parental habitat [52–54]. Suggested by this study, larvae from Taiwan may not have a longer dispersal distance because their larger size did not correspond to larger stores of long-term energy biomolecules (WE).
(b). Responses of larval lipid consumption and other physiological traits to changes in pCO2 and temperature
Changes in energy stores of coral larvae under future ocean change may affect coral populations through shifts in dispersal distance and recruitment success. TG can be quickly hydrolysed for immediate energy needs and is the first lipid class to be consumed during larval development or during periods of starvation (e.g. [55]). WE has a slower turnover rate and serves as long-term energy deposits for larvae [8,56,57]. In addition, WE plays an important role in dispersal of coral larvae, governing buoyancy (e.g. [57]) and entraining larvae in surface currents [58]. If TG stores are consumed, larvae will use WE, but their vertical position in the water column will change as a result, with potentially negative consequences for dispersal.
Additionally, Symbiodinium dinoflagellates in brooded larvae, vertically transmitted to larvae prior to release, satisfy a significant portion of larval energy budgets [12]. Changes in lipid content of planulae, like P. damicornis, represent the balance between demands of holobiont metabolism and the carbon translocation by Symbiodinium. In particular, WE consumption increases when endosymbiont metabolites are not available [12].
When lipid utilization was quantified in P. damicornis planulae over 24 h under experimental conditions, a range of responses across treatments and biogeographic sites was observed. For example, in Moorea at High-T, High-pCO2, planulae depleted up to half of their energy-storage lipids while in Taiwan at Ambient-T, Ambient-pCO2, other planulae more than doubled their deposits of these lipids. Assuming constant rates of respiration over a 24-h period (Peak larvae, Moorea, approx. 0.08–0.13 nmol O2 per larva per min [6]), larvae would need to consume 1.33–2.16 µg WE over the incubation [59]. Larvae in this study consumed less than or equal to 1 µg WE per symbiont density, but photosynthetic activities and consumption of TG may have accounted for the remaining energy burned during respiration. The net production of WE and TG during experimental exposures was likely due to the photosynthetic activities of Symbiodinium. Given the ecological significance that energy-storage lipids play in the successful dispersal of these lecithotrophic larvae [8–10], the point when larvae stop accumulating lipid and start depleting lipid stores may be a useful indicator of stressful environmental conditions in future work.
Higher temperatures, but not pCO2, may affect larval dispersal for some populations in the future, due to changes in WE that alter buoyancy of planulae (e.g. [57]). For Peak planulae in this study, WE content was sensitive to temperature, depending on the site, but not to pCO2 (figure 1). In Taiwan, under Ambient-T, WE content increased over the incubation period, while larvae at High-T experienced net depletion. Metabolic rates of planulae increase with temperature [4,6], so utilization of lipids, from stores or from photosynthesis, would also be expected to rise. However, in Moorea, WE content of larvae did not respond to changes in temperature. Increased primary productivity of Symbiodinium at High-T or increased rates of translocation may explain this pattern, as P. damicornis in Moorea tend to host a more thermotolerant Symbiodinium clade [50,51] than P. damicornis in Taiwan [49]. Putnam et al. [60] observed no change in the photophysiology of P. damicornis planulae in response to elevated pCO2 and temperature; however, rates of translocation of fixed carbon can increase in response to low pH and elevated temperature [61,62]. Translocated carbon could be used to satisfy immediate energy demands rather than oxidation of stored WE [12].
The utilization of WE may occur when demand for energy surpasses the pool of TG (e.g. [63]). In general, TG content remained constant with respect to temperature and pCO2 levels. Differences in TG content between sites followed the pattern of maternal investment of this energy-storage lipid. In Moorea, the energetic demands of early dispersal surpassed the combined resources of TG synthesis and translocated fixed carbon, perhaps due to smaller initial TG stores for larvae from Moorea (figure 1). The smaller pool of TG was likely partially maintained through oxidation of WE [64,65]. In Taiwan, planulae had higher TG content, particularly under Ambient-T. Larvae from Taiwan may contain a surplus of TG, due to higher maternal investment. Conversion of TG to WE under Ambient-T may have caused the increase in WE observed under these conditions. Overall, the species-level patterns of lipid utilization and production indicate that P. damicornis planulae are launching a physiological response to conditions of warming, but not OA, and this response is at least partially fuelled by the utilization of WE and TG. As a result, planulae store less lipid under warming conditions, particularly planulae in Taiwan, and therefore may have fewer resources for performing the developmental changes associated with metamorphosis and settlement (e.g. [66]).
Our measurements of utilization of energy-storage lipids represent the net activity of the planula holobiont (animal + symbiont). The symbiont cells themselves contain lipid that contributes to the measured amount for the holobiont (e.g. [67]). Because symbiont density did not vary between sites, pCO2 levels or temperature levels, differences in function of the Symbiodinium, in addition to changes in host physiology, may have contributed to patterns of lipid utilization and production (figure 1). The photosynthetic activities of Symbiodinium as well as their translocation rates of glycerol and lipid bodies containing WE and TG potentially change the amount of fixed carbon received by the host [68,69] and therefore the amount of lipid available.
Traits of size and growth were not consistently significantly affected by elevated pCO2 and temperature. Planula size in P. damicornis may not be uniformly sensitive to OA and warming due to the contribution of Symbiodinium to larval energy metabolism; this input of exogenous energy may even allow larvae to grow in size during dispersal, a possibility not available to aposymbiotic lecithotrophic (non-feeding) larvae. Firstly, PL levels were higher at warmer temperatures, but only for larvae in Moorea. Secondly, larval area and length were greater for larvae released in Moorea versus Taiwan, though this pattern differed from that of PL. If this trend of reduced planula size over the first 24 h of dispersal in Taiwan continues throughout the larval stage, it may carry over to reduce the stringency of habitat selection for settlement, increase post-settlement survival and growth, and increase the age of first reproduction [52,70,71]. However, even though larval size decreased over the 24-h exposures in Taiwan, absolute larval size still remained larger than larvae in Moorea. While smaller planula size at the beginning of settlement would be a fitness disadvantage [72,73], this may not be the case for planulae from Taiwan, which maintained their symbiont density, TL and TP, despite the decrease in larval size.
(c). The role of environmental history and geographical site
The study sites in Moorea and Taiwan had different regimes of temperature and pH [32] that have presumably persisted over the recent history of the study populations. Corals in Moorea experienced, on average, warmer temperatures with a narrower range of values. On the other hand, corals in Taiwan experienced lower mean pH and a greater range of pH values. In both sites, autonomous sensors deployed on natal fringing reefs [32] confirmed that the Ambient treatment conditions were observed within the water mass bathing the fringing reef during the experiment, while the High treatment conditions were not. Overlaid on the between-site differences in environmental conditions are the genetic differences between P. damicornis populations [74,75], which may indicate the possibility of local adaptation to environmental conditions. Although not predictive, our results shed light on the possibility that the physiological plasticity of P. damicornis planulae to resist future ocean conditions may be influenced by the environmental conditions to which its population is adapted or acclimatized [19]. In general, our results can be used to evaluate some expectations based on a local adaptation scenario: planulae from Moorea should perform better than those from Taiwan under High-T, and planulae from Taiwan should perform better than those from Moorea under High-pCO2. In support, for example, we found that larvae from Taiwan were more sensitive to temperature, producing fewer energy-storage lipids under High-T. In Moorea, planulae at High-T consumed similar amounts of lipid as at Ambient-T, suggesting that their demands for stored energy were not elevated, perhaps offset by increased carbon translocation from their symbionts. Contrary to expectations from a local adaptation scenario, in general, planulae in Moorea and Taiwan responded similarly to pCO2. Additionally, corals in the study sites with different environments produced larvae with different characteristics, which may play a role in the different physiological responses observed.
5. Conclusion
Comparisons of physiological responses of P. damicornis larvae to OA and warming between sites across the species' biogeographic range improve our understanding of the possible future success of this species. An overarching outcome of this study is that site-specific aspects of planula physiology provide a framework for describing and understanding the consequences of environmental stress. For example, the effects of temperature on WE, long-term energy-storage lipids with an important role in larval buoyancy, differed based on biogeographic site. Because larvae from Moorea are endowed with more total lipid and thus have higher absolute amounts of WE upon release, they may be able to accommodate depletion of these stores without sacrificing their dispersal potential or the energy reserves required to complete metamorphosis and settlement. Our results support expectations for performance based on a local adaptation scenario. While there is still much to learn about the interplay of environmental history and population genetics, the variety of physiological responses maintained within P. damicornis may enhance the overall persistence of this species in the light of global climate change.
Supplementary Material
Acknowledgements
A portion of this research was conducted at the Richard B. Gump South Pacific Research Station (UC Berkeley) in collaboration with the Moorea Coral Reef Long-Term Ecological Research (MCR LTER) programme. Another portion of this research was conducted at the National Museum of Marine Biology and Aquarium in Taiwan. We thank Dr Peter Edmunds and members of his research group for his support of this work. We thank Dr Brian Rivest for assistance with experiments and fieldwork in Moorea, as well as Keith Seydel, Vince Moriarty, Dr Steeve Comeau, Nate Spindel, Chelsea Behymer and Gump Station staff. We are grateful for the support of Drs Mark Ohman and Aaron Hartmann in facilitating lipid analyses of Moorea samples. We thank Song Shin-Ni and Chen Hung-Kai for valuable laboratory assistance at NMMBA. We also thank Ray Tarn and Wei-Jei, who helped coordinate field deployments and collections in Taiwan. Many thanks to undergraduates Katrina Shao, Silke Bachhuber, Farallon Broughton, Fiona Luong, Yana Nebuchina, Lu Raymond, Lin Yuan-Jheng and Cheng Ya-Wen for assistance with sample analysis. Finally, we acknowledge Dr Daniel Okamoto for assistance with statistical analyses.
Data accessibility
The biological and environmental datasets supporting this article are publicly available in the LTER Network Data Catalog (portal.lternet.edu) as well as the MCR LTER local data catalogue, with the identifier knb-lter-mcr.5022.
Authors' contributions
E.B.R. conceived of the study, designed the study, carried out the experiments and associated laboratory sample analyses, carried out the statistical analyses and drafted the manuscript. C.-S.C. and T.-Y.F. helped coordinate the study. H.-H.L. participated in the design of the lipid analysis and helped coordinate the study. G.E.H. helped design and coordinate the study and helped draft the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
During the course of this research, E.B.R. was supported by a National Science Foundation Graduate Research Fellowship as well as a graduate fellowship from the Department of Ecology, Evolution and Marine Biology at UC Santa Barbara. A mini-grant from the MCR LTER to E.B.R. and G.E.H. funded the majority of the work in Moorea. Additionally, the MCR LTER provided boating and SCUBA diving resources and use of experimental and laboratory facilities. An award from the National Science Foundation Doctoral Dissertation Enhancement Program to E.B.R. funded the majority of the work in Taiwan.
References
- 1.Albright R, Mason B, Miller M, Langdon C. 2010. Ocean acidification compromises recruitment success of the threatened Caribbean coral Acropora palmata. Proc. Natl Acad. Sci. USA 107, 20 400–20 404. ( 10.1073/pnas.1007273107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albright R, Langdon C. 2011. Ocean acidification impacts multiple early life history processes of the Caribbean coral Porites astreoides. Glob. Chang. Biol. 17, 2478–2487. ( 10.1111/j.1365-2486.2011.02404.x) [DOI] [Google Scholar]
- 3.Suwa R, Nakamura M, Morita M. 2010. Effects of acidified seawater on early life stages of scleractinian corals (Genus Acropora). Fish. Sci. 76, 93–99. ( 10.1007/s12562-009-0189-7) [DOI] [Google Scholar]
- 4.Cumbo VR, Edmunds PJ, Wall CB. 2013. Brooded coral larvae differ in their response to high temperature and elevated pCO2 depending on the day of release. Mar. Biol. 160, 2903–2917. ( 10.1007/s00227-013-2280-y) [DOI] [Google Scholar]
- 5.Cumbo VR, Fan T-Y, Edmunds PJ. 2013. Effects of exposure duration on the response of Pocillopora damicornis larvae to elevated temperature and high pCO2. J. Exp. Mar. Bio. Ecol. 439, 100–107. ( 10.1016/j.jembe.2012.10.019) [DOI] [Google Scholar]
- 6.Rivest EB, Hofmann GE. 2014. Responses of the metabolism of the larvae of Pocillopora damicornis to ocean acidification and warming. PLoS ONE 9, e96172 ( 10.1371/journal.pone.0096172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chua C-M, Leggat W, Moya A. 2013. Near-future reductions in pH will have no consistent ecological effects on the early life-history stages of reef corals. Mar. Ecol. Prog. Ser. 486, 143–151. ( 10.3354/meps10318) [DOI] [Google Scholar]
- 8.Richmond RH. 1987. Energetics, competence, and long-distance dispersal of planula larvae of the coral Pocillopora damicornis. Mar. Biol. 93, 527–533. ( 10.1007/bf00392790) [DOI] [Google Scholar]
- 9.Harii S, Kayanne H, Takigawa H, Hayashibara T, Yamamoto M. 2002. Larval survivorship, competency periods and settlement of two brooding corals, Heliopora coerulea and Pocillopora damicornis. Mar. Biol. 141, 39–46. ( 10.1007/s00227-002-0812-y) [DOI] [Google Scholar]
- 10.Harii S, Nadaoka K, Yamamoto M. 2007. Temporal changes in settlement, lipid content and lipid composition of larvae of the spawning hermatypic coral Acropora tenuis. Mar. Ecol. Prog. Ser. 346, 89–96. ( 10.3354/meps07114) [DOI] [Google Scholar]
- 11.Vavra J, Manahan DT. 1999. Protein metabolism in lecithotrophic larvae (Gastropoda: Haliotis rufescens). Biol. Bull. 196, 177–186. ( 10.2307/1542563) [DOI] [PubMed] [Google Scholar]
- 12.Harii S, Yamamoto M, Hoegh-Guldberg O. 2010. The relative contribution of dinoflagellate photosynthesis and stored lipids to the survivorship of symbiotic larvae of the reef-building corals. Mar. Biol. 157, 1215–1224. ( 10.1007/s00227-010-1401-0) [DOI] [Google Scholar]
- 13.Richmond RH. 1982. Energetic considerations in the dispersal of Pocillopora damicornis (Linnaeus) planulae. In Proc. of the 4th Int. Coral Reef Symp., Manila, Phillippines, 18–22 May 1981, vol. 2, pp. 153–156.
- 14.Edmunds PJ, Gates RD, Gleason DF. 2001. The biology of larvae from the reef coral Porites astreoides, and their response to temperature disturbances. Mar. Biol. 139, 981–989. ( 10.1007/s002270100634) [DOI] [Google Scholar]
- 15.Kempf S. 1981. Long-lived larvae of the gastropod Aplysia juliana: do they disperse and metamorphose or just slowly fade away? Mar. Ecol. Prog. Ser. 6, 61–65. ( 10.3354/meps006061) [DOI] [Google Scholar]
- 16.Lucas MI, Walker G, Holland DL, Crisp DJ. 1979. An energy budget for the free-swimming and metamorphosing larvae of Balanus balanoides (Crustacea: Cirripedia). Mar. Biol. 55, 221–229. ( 10.1007/BF00396822) [DOI] [Google Scholar]
- 17.Szmant AM, Meadows MG. 2006. Developmental changes in coral larval buoyancy and vertical swimming behavior: implications for dispersal and connectivity. In Proc. 10th Int. Coral Reef Symp., pp. 431–437.
- 18.Kawecki TJ, Ebert D. 2004. Conceptual issues in local adaptation. Ecol. Lett. 7, 1225–1241. ( 10.1111/j.1461-0248.2004.00684.x) [DOI] [Google Scholar]
- 19.Kelly MW, Hofmann GE. 2012. Adaptation and the physiology of ocean acidification. Funct. Ecol. 27, 980–990. ( 10.1111/j.1365-2435.2012.02061.x) [DOI] [Google Scholar]
- 20.Alleaume-Benharira M, Pen I, Ronce O. 2006. Geographical patterns of adaptation within a species’ range: interactions between drift and gene flow. J. Evol. Biol. 19, 203–215. ( 10.1111/j.1420-9101.2005.00976.x) [DOI] [PubMed] [Google Scholar]
- 21.Conover DO. 1998. Local adaptation in marine fishes: evidence and implications for stock enhancement. Bull. Mar. Sci. 62, 477–493. [Google Scholar]
- 22.Sanford E, Kelly MW. 2011. Local adaptation in marine invertebrates. Annu. Rev. Mar. Sci. 3, 509–535. ( 10.1146/annurev-marine-120709-142756) [DOI] [PubMed] [Google Scholar]
- 23.Kelly MW, Padilla-Gamiño JL, Hofmann GE. 2013. Natural variation and the capacity to adapt to ocean acidification in the keystone sea urchin Strongylocentrotus purpuratus. Glob. Chang. Biol. 19, 2536–2546. ( 10.1111/gcb.12251) [DOI] [PubMed] [Google Scholar]
- 24.Hofmann GE, et al. 2014. Exploring local adaptation and the ocean acidification seascape—studies in the California Current Large Marine Ecosystem. Biogeosciences 11, 1053–1064. ( 10.5194/bg-11-1053-2014) [DOI] [Google Scholar]
- 25.Kroeker KJ, et al. 2016. Interacting environmental mosaics drive geographic variation in mussel performance and predation vulnerability. Ecol. Lett. 19, 771–779. ( 10.1111/ele.12613) [DOI] [PubMed] [Google Scholar]
- 26.Oliver TA, Palumbi SR. 2011. Do fluctuating temperature environments elevate coral thermal tolerance? Coral Reefs 30, 429–440. ( 10.1007/s00338-011-0721-y) [DOI] [Google Scholar]
- 27.Sultan SE. 2007. Development in context: the timely emergence of eco-devo. Trends Ecol. Evol. 22, 575–582. ( 10.1016/j.tree.2007.06.014) [DOI] [PubMed] [Google Scholar]
- 28.Lee H-J, Chao S-Y, Fan K-L, Kuo T-Y. 1999. Tide-induced eddies and upwelling in a semi-enclosed basin: Nan Wan. Estuar. Coast. Shelf Sci. 49, 775–787. ( 10.1006/ecss.1999.0524) [DOI] [Google Scholar]
- 29.Rivest EB, Hofmann GE. 2015. Effects of temperature and pCO2 on lipid use and biological parameters of planulae of Pocillopora damicornis. J. Exp. Mar. Bio. Ecol. 473, 43–52. ( 10.1016/j.jembe.2015.07.015) [DOI] [Google Scholar]
- 30.Fan T-Y, Lin K-H, Kuo F-W, Soong K, Liu L-L, Fang L-S. 2006. Diel patterns of larval release by five brooding scleractinian corals. Mar. Ecol. Prog. Ser. 321, 133–142. ( 10.3354/meps321133) [DOI] [Google Scholar]
- 31.Cumbo VR, Baird AH, van Oppen MJH. 2013. The promiscuous larvae: flexibility in the establishment of symbiosis in corals. Coral Reefs 32, 111–120. ( 10.1007/s00338-012-0951-7) [DOI] [Google Scholar]
- 32.Rivest EB, Gouhier TC. 2015. Complex environmental forcing across the biogeographical range of coral populations. PLoS ONE 10, e0121742 ( 10.1371/journal.pone.0121742) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leichter J. 2014. MCR LTER: Coral reef: benthic water temperature, ongoing since 2005. Moorea Coral Reef LTER. Long Term Ecol. Res. Netw.
- 34.IPCC. 2013. Summary for policymakers. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds Stocker T, Qin D, Plattner G-K, Tignor M, Allen S, Boschung J, Nauels A, Xia Y, Bex V, Midgley P). Cambridge, UK: Cambridge University Press. [Google Scholar]
- 35.Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917. ( 10.1139/o59-099) [DOI] [PubMed] [Google Scholar]
- 36.Luo YJ. 2008. Lipid bodies in the marine endosymbiosis. MSc thesis, National Tsing Hwa University, Taiwan.
- 37.Rouser G, Fleischer S, Yamamoto A. 1970. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 5, 494–496. ( 10.1007/BF02531316) [DOI] [PubMed] [Google Scholar]
- 38.Bradford MM. 1976. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 72, 248–254. ( 10.1006/abio.1976.9999) [DOI] [PubMed] [Google Scholar]
- 39.Jaeckle WB, Manahan DT. 1989. Growth and energy imbalance during the development of a lecithotrophic molluscan larva (Haliotis rufescens). Biol. Bull. 177, 237–246. ( 10.2307/1541939) [DOI] [Google Scholar]
- 40.Rasband WS. 1997. ImageJ. Bethesda, MD: US National Institutes of Health. See http://imagej.nih.gov/ij.
- 41.Isomura N, Nishihira M. 2001. Size variation of planulae and its effect on the lifetime of planulae in three pocilloporid corals. Coral Reefs 20, 309–315. ( 10.1007/s003380100180) [DOI] [Google Scholar]
- 42.Pinheiro JC, Bates DM. 2000. Mixed-effects models in S and S-PLUS Springer. New York, NY: Springer. [Google Scholar]
- 43.Burnham KP, Anderson DR. 2002. Model selection and multi-model inference: a practical information-theoretic approach. New York, NY: Springer. [Google Scholar]
- 44.Crawley MJ. 2013. The R book, 2nd edn West Sussex, UK: John Wiley & Sons. [Google Scholar]
- 45.Zuur AF, Ieno E, Walker N, Saveliev A, Smith G. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer. [Google Scholar]
- 46.Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biometrical J. 50, 346–363. ( 10.1002/bimj.200810425) [DOI] [PubMed] [Google Scholar]
- 47.Adjeroud M, Guérécheau A, Vidal-Dupiol J, Flot J-F, Arnaud-Haond S, Bonhomme F. 2013. Genetic diversity, clonality and connectivity in the scleractinian coral Pocillopora damicornis: a multi-scale analysis in an insular, fragmented reef system. Mar. Biol. 161, 531–541. ( 10.1007/s00227-013-2355-9) [DOI] [Google Scholar]
- 48.Torda G, Lundgren P, Willis BL, Van Oppen MJH. 2013. Genetic assignment of recruits reveals short- and long-distance larval dispersal in Pocillopora damicornis on the Great Barrier Reef. Mol. Ecol. 22, 5821–5834. ( 10.1111/mec.12539) [DOI] [PubMed] [Google Scholar]
- 49.Chen CA, Yang Y-W, Wei NV, Tsai W-S, Fang L-S. 2004. Symbiont diversity in scleractinian corals from tropical reefs and subtropical non-reef communities in Taiwan. Coral Reefs 24, 11–22. ( 10.1007/s00338-004-0389-7) [DOI] [Google Scholar]
- 50.Putnam HM, Stat M, Pochon X, Gates RD. 2012. Endosymbiotic flexibility associates with environmental sensitivity in scleractinian corals. Proc. R. Soc. B 279, 4352–4361. ( 10.1098/rspb.2012.1454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stat M, Gates RD. 2011. Clade D Symbiodinium in scleractinian corals: a ‘nugget’ of hope, a selfish opportunist, an ominous sign, or all of the above. J. Mar. Biol. 2011, 730715 ( 10.1155/2011/730715) [DOI] [Google Scholar]
- 52.Marshall DJ, Keough MJ. 2003. Variation in the dispersal potential of non-feeding invertebrate larvae: the desperate larva hypothesis and larval size. Mar. Ecol. Prog. Ser. 255, 145–153. ( 10.3354/meps255145) [DOI] [Google Scholar]
- 53.Morgan SG. 1995. The timing of larval release. In Ecology of marine invertebrate larvae (ed. McEdward L.), pp. 157–191. Boca Raton, FL: CRC Press. [Google Scholar]
- 54.Pechenik JA. 1999. On the advantages and disadvantages of larval stages in benthic marine invertebrate life cycles. Mar. Ecol. Prog. Ser. 177, 269–297. ( 10.3354/meps177269) [DOI] [Google Scholar]
- 55.Sewell MA. 2005. Utilization of lipids during early development of the sea urchin Evechinus chloroticus. Mar. Ecol. Prog. Ser. 304, 133–142. ( 10.3354/meps304133) [DOI] [Google Scholar]
- 56.Villinski JT, Villinski JC, Byrne M, Raff RA. 2002. Convergent maternal provisioning and life-history evolution in echinoderms. Evolution 56, 1764–1775. ( 10.1111/j.0014-3820.2002.tb00190.x) [DOI] [PubMed] [Google Scholar]
- 57.Lee RF, Hagen W, Kattner G. 2006. Lipid storage in marine zooplankton. Mar. Ecol. Prog. Ser. 307, 273–306. ( 10.3354/meps307273) [DOI] [Google Scholar]
- 58.Willis BL, Oliver JK. 1990. Direct tracking of coral larvae: implications for dispersal studies of planktonic larvae in topographically complex environments. Ophelia 32, 145–162. ( 10.1080/00785236.1990.10422029) [DOI] [Google Scholar]
- 59.Davies PS. 1991. Effect of daylight variations on the energy budgets of shallow-water corals. Mar. Biol. 108, 137–144. ( 10.1007/BF01313481) [DOI] [Google Scholar]
- 60.Putnam HM, Mayfield AB, Fan T-Y, Chen C-S, Gates RD. 2013. The physiological and molecular responses of larvae from the reef-building coral Pocillopora damicornis exposed to near-future increases in temperature and pCO2. Mar. Biol. 160, 2157–2173. ( 10.1007/s00227-012-2129-9) [DOI] [Google Scholar]
- 61.Tremblay P, Fine M, Maguer JF, Grover R, Ferrier-Pagès C. 2013. Photosynthate translocation increases in response to low seawater pH in a coral–dinoflagellate symbiosis. Biogeosciences 10, 3997–4007. ( 10.5194/bgd-10-83-2013) [DOI] [Google Scholar]
- 62.Loram JE, Trapido-Rosenthal HG, Douglas AE. 2007. Functional significance of genetically different symbiotic algae Symbiodinium in a coral reef symbiosis. Mol. Ecol. 16, 4849–4857. ( 10.1111/j.1365-294X.2007.03491.x) [DOI] [PubMed] [Google Scholar]
- 63.Sargent JR, Gatten RR, McIntosh R. 1977. Wax esters in the marine environment—their occurrence, formation, transformation and ultimate fates. Mar. Chem. 5, 573–584. ( 10.1016/0304-4203(77)90043-3) [DOI] [Google Scholar]
- 64.Patton JS, Benson AA. 1975. A comparative study of wax ester digestion in fish. Comp. Biochem. Physiol. B Comp. Biochem. 52, 111–116. ( 10.1016/0305-0491(75)90125-X) [DOI] [PubMed] [Google Scholar]
- 65.Bauermeister AEM, Sargent JR. 1979. Biosynthesis of triacylglycerols in the intestines of rainbow trout (Salmo gairdnerii) fed marine zooplankton rich in wax esters. Biochim. Biophys. Acta - Lipids Lipid Metab. 575, 358–364. ( 10.1016/0005-2760(79)90104-8) [DOI] [PubMed] [Google Scholar]
- 66.Gallager SM, Mann R, Sasaki GC. 1986. Lipid as an index of growth and viability in three species of bivalve larvae. Aquaculture 56, 81–103. ( 10.1016/0044-8486(86)90020-7) [DOI] [Google Scholar]
- 67.Harland AD, Fixter LM, Spencer Davies P, Anderson RA. 1991. Distribution of lipids between the zooxanthellae and animal compartment in the symbiotic sea anemone Anemonia viridis: wax esters, triglycerides and fatty acids. Mar. Biol. 110, 13–19. ( 10.1007/BF01313087) [DOI] [Google Scholar]
- 68.Muscatine L. 1967. Glycerol excretion by symbiotic algae from corals and tridacna and its control by the host. Science 156, 516–519. ( 10.1126/science.156.3774.516) [DOI] [PubMed] [Google Scholar]
- 69.Chen W-NU, Kang H-J, Weis VM, Mayfield AB, Jiang P-L, Fang L-S, Chen C-S. 2012. Diel rhythmicity of lipid-body formation in a coral–Symbiodinium endosymbiosis. Coral Reefs 31, 521–534. ( 10.1007/s00338-011-0868-6) [DOI] [Google Scholar]
- 70.Miller S. 1993. Larval period and its influence on post-larval life history: comparison of lecithotrophy and facultative planktotrophy in the aeolid nudibranch Phestilla sibogae. Mar. Biol. 117, 635–645. ( 10.1007/BF00349776) [DOI] [Google Scholar]
- 71.Woollacott RM, Pechenik JA, Imbalzano KM. 1989. Effects of duration of larval swimming period on early colony development in Bugula stolonifera (Bryozoa: Cheilostomata). Mar. Biol. 102, 57–63. ( 10.1007/BF00391323) [DOI] [Google Scholar]
- 72.Bernardo J. 1996. The particular maternal effect of propagule size, especially egg size: patterns, models, quality of evidence and interpretations. Am. Zool. 36, 216–236. ( 10.1093/icb/36.2.216) [DOI] [Google Scholar]
- 73.Stearns SC. 1992. The evolution of life histories. Oxford, UK: Oxford University Press. [Google Scholar]
- 74.Schmidt-Roach S, Miller KJ, Lundgren P, Andreakis N. 2014. With eyes wide open: a revision of species within and closely related to the Pocillopora damicornis species complex (Scleractinia; Pocilloporidae) using morphology and genetics. Zool. J. Linn. Soc. 170, 1–33. ( 10.1111/zoj.12092) [DOI] [Google Scholar]
- 75.Forsman ZH, Johnston EC, Brooks AJ, Adam TC, Toonen RJ. 2013. Genetic evidence for regional isolation of Pocillopora corals from Moorea. Oceanography 26, 153–155. ( 10.5670/oceanog.2013.58) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The biological and environmental datasets supporting this article are publicly available in the LTER Network Data Catalog (portal.lternet.edu) as well as the MCR LTER local data catalogue, with the identifier knb-lter-mcr.5022.