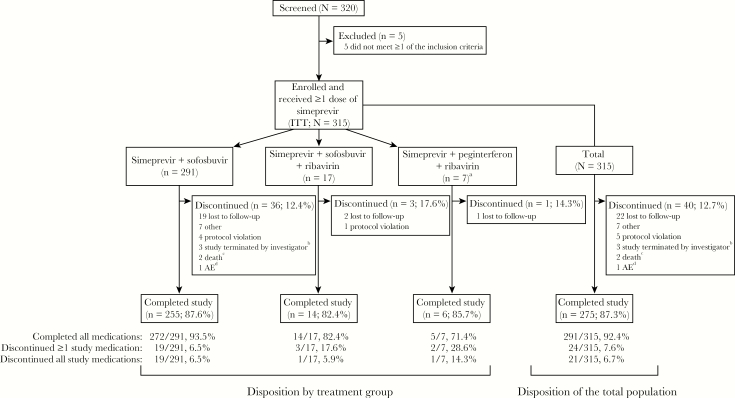
Figure 1.
Patient disposition. aData for the simeprevir + peginterferon + ribavirin group are not reported due to the small sample size; however, data for these patients are included in the total population. bPatients were discontinued when the investigator decided to no longer participate in the study. cBoth deaths occurred >30 days after the last dose of study medication and were considered unrelated to simeprevir. dAdverse event (AE) was grade 3 thrombocytopenia and was considered very likely related to simeprevir and sofosbuvir. ITT, intent to treat.