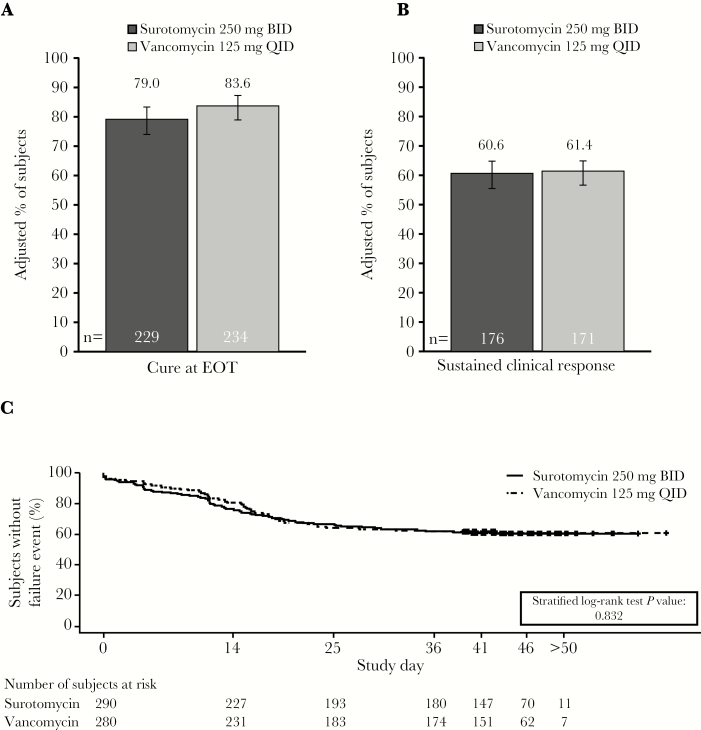
Figure 3.
Clinical outcomes according to treatment (microbiological modified intent to treat population). (A) Clinical cure rate at EOT (Day 10), (B) sustained clinical response at the end of the trial (Day 40–50), and (C) Kaplan-Meier analysis of clinical response over time. The estimated adjusted proportions were weighted averages across all strata, constructed using Mehrotra-Railkar continuity-corrected minimum-risk stratum weights. Sustained clinical response was defined as a subject deemed a cure at EOT who did not have recurrence, did not die, and was not lost to follow-up. BID, twice daily; EOT, end of treatment; QID, 4 times daily.