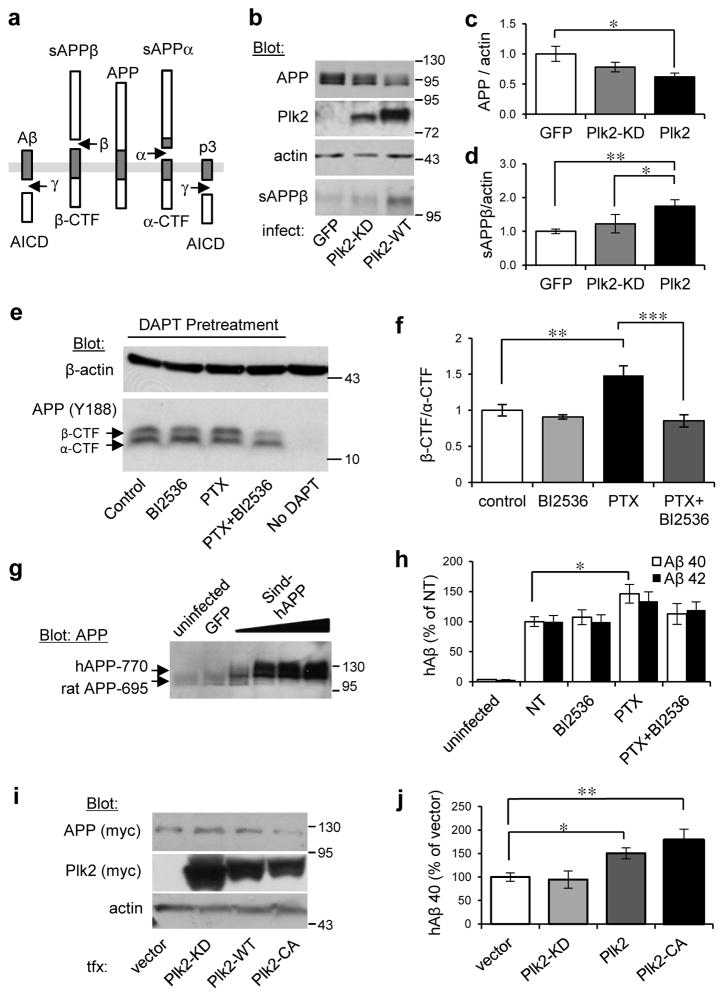
Fig. 3. Plk2 stimulates APP β-processing.
(a) Schematic of APP processing pathways. (b) Hippocampal cultured neurons (21 DIV) were infected with Sindbis viruses for 18 hr as shown at bottom and lysates analyzed by immunoblotting for proteins indicated at left. (c,d) Quantification of (c) full-length APP and (d) sAPPβ from b, normalized to actin and GFP (for full-length APP: n=9 independent cultures for GFP, 11 for Plk2-KD, 18 for Plk2-WT; for sAPPβ: n=6 cultures for each group). (e) Hippocampal neurons (DIV 19–20) were treated with PTX or vehicle and co-treated with BI2536 (50 nM) as indicated, then immunolabeled with rabbit monoclonal APP-C antibodies (Y188). Neurons were pretreated with γ-secretase inhibitor (DAPT, 1 μm) 30 min prior to stimulation in order to prevent CTF degradation, which confirms the specificity of β- and α-CTF bands (note absence of CTFs without DAPT). (f) Quantification β-/α-CTF ratio from e (n=5 cultures). (g) Neurons were uninfected or infected with Sindbis-hAPP770 or GFP. Human APP was detected by immunoblotting with APP-N antibodies (exogenous hAPP770 appears larger than the dominant endogenous rat APP695 form). (h) Sindbis-hAPP-infected or uninfected neurons were treated with PTX (25 μM, 20 h) or vehicle (NT) and co-treated with BI2536 (50 nM) or vehicle (DMSO). Human Aβ40/42 (hAβ) were measured in conditioned media by species-specific ELISA and normalized against hAPP expression levels from immunoblotting (n=2 cultures for uninfected, 6 for NT, 6 for BI2536, 5 for PTX, 6 for PTX+BI2536). (i) N2a-myc-hAPP-Swe cells were transfected as indicated and lysates analyzed by immunoblotting. (j) Quantification of hAβ40 in conditioned media from i (n=7 cultures for vector, 8 for Plk2-KD, 8 for Plk2-WT, 9 for Plk2-CA). ***P<0.001, **P<0.01, *P<0.05; ANOVA with Tukey’s post hoc test. Data are means±SEM. Experiments were performed in at least duplicate. Molecular weights are kDa. Cropped blots are displayed for clear and concise presentation. Full western blots are shown in Supplementary Fig. 10.