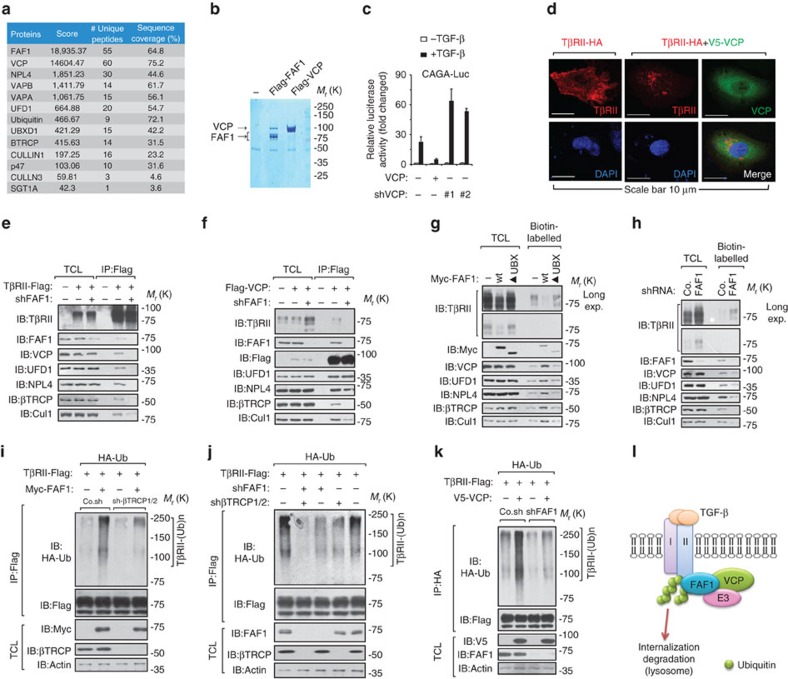
Figure 3. FAF1 recruits VCP/βTRCP to target TβRII for polyubiquitination.
(a) Mass spectrometry studies of anti-Flag-FAF1 immunoprecipitants revealed FAF1-binding partners. (b) FAF1 bound to VCP at a ratio close to 1:1. (c) The CAGA12-Luc SMAD-dependent transcriptional response of HEK293T cells expressing VCP or VCP shRNA (#1 and #2) and treated with TGF-β (2.5 ng ml−1) overnight is shown. The data are presented as the mean±s.d. of three independent sets of experiments. (d) Immunofluorescence and 4, 6-diamidino-2-phenylindole (DAPI) staining of HeLa cells transfected with TβRII-HA or TβRII-HA with V5-VCP plasmids. Scale bar, 10 μm. (e,f) HEK293T cells were transfected with TβRII-Flag (e) or Flag-VCP (f) and with FAF1 depletion or not, as indicated. Cells were collected for immunoprecipitation (IP) and immunoblot (IB) analysis; 5% of the total cell lysate was loaded as an input. (g,h) MDA-MB-231 cells expressing Myc-FAF1 wt/UBX-deletion mutant (▴UBX) (g) or infected with FAF1 shRNA as indicated (h) were harvested for biotinylation and immunoblot (IB) analysis. Co., control non-targeting shRNA. (i) HA-Ub stably expressing HEK293T cells were transfected with TβRII-Flag along with Myc-FAF1 and were infected with control (Co.sh) or βTRCP shRNA lentivirus as indicated. The cells were then collected for IP and IB analysis. (j) HA-Ub stably expressing HEK293T cells were transfected with TβRII-Flag and infected with βTRCP1/2 shRNA and/or FAF1 shRNA lentivirus as indicated. Cells were then collected for IP and IB analysis. (k) HA-Ub stably expressed HEK293T cells were transfected with TβRII-Flag and VCP, and were infected with FAF1 shRNA lentivirus as indicated. Cells were then collected for IP and IB analysis. (l) Working model of how FAF1 mediates TβRII polyubiquitylation and turnover via lysosomal mediated degradation.