Abstract
Background
The objective of this analysis was to evaluate the efficacy and safety of oritavancin compared with vancomycin for patients with acute bacterial skin and skin structure infections (ABSSSIs) who received treatment in the outpatient setting in the Phase 3 SOLO clinical trials.
Methods
SOLO I and SOLO II were 2 identically designed comparative, multicenter, double-blind, randomized studies to evaluate the efficacy and safety of a single 1200-mg dose of intravenous (IV) oritavancin versus 7–10 days of twice-daily IV vancomycin for the treatment of ABSSSI. Protocols were amended to allow enrolled patients to complete their entire course of antimicrobial therapy in an outpatient setting. The primary efficacy outcome was a composite endpoint (cessation of spread or reduction in size of the baseline lesion, absence of fever, and no rescue antibiotic at early clinical evaluation [ECE]) (48 to 72 hours). Key secondary endpoints included investigator-assessed clinical cure 7 to 14 days after end of treatment (posttherapy evaluation [PTE]) and 20% or greater reduction in lesion area at ECE. Safety was assessed until day 60.
Results
Seven hundred ninety-two patients (oritavancin, 392; vancomycin, 400) received entire course of treatment in the outpatient setting. Efficacy response rates at ECE and PTE were similar (primary composite endpoint at ECE: 80.4% vs 77.5% for oritavancin and vancomycin, respectively) as was incidence of adverse events. Five patients (1.3%) who received oritavancin and 9 (2.3%) vancomycin patients were subsequently admitted to a hospital.
Conclusions
Oritavancin provides a single-dose alternative to multidose vancomycin for treatment of ABSSSI in the outpatient setting.
Keywords: ABSSSI, long-acting lipoglycopeptides, oritavancin, skin infections, vancomycin
Despite advances in medical care, acute bacterial skin and skin structure infections (ABSSSIs) remain one of the leading causes of infection-related emergency room visits and hospital admissions [1]. In the United States alone, it is estimated that there are over 3 million emergency room visits and 870000 hospital admissions annually due to ABSSSIs [1, 2]. Care of patients with ABSSSI places a major financial burden on the US healthcare system, largely due to hospitalization costs. On average, inpatient treatment costs range between $6000 and $10000 [3]. Data also suggest that a substantial portion of patients with ABSSSI are unnecessarily admitted to the hospital, because most admissions occur among patients in whom comorbidities are absent or few and no systemic signs and symptoms of infection are present [4]. The administration of multiday intravenous (IV) antibiotics constitute the primary reason for admission for many patients [5]. Outpatient treatment of ABSSSI has been estimated to save $2500 to $6500 per patient [3]. Given current US healthcare expenditures for ABSSSIs, it is critical to identify therapies that can safely and effectively shift care from the inpatient to the outpatient setting while ensuring that care can be delivered with minimal patient visits, healthcare resources, and subsequent hospital admissions.
Oritavancin (Orbactiv; The Medicines Company, Parsippany, NJ) is a recently approved IV semisynthetic lipoglycopeptide antibiotic indicated for the treatment of adult patients with ABSSSI caused by designated Gram-positive pathogens, including methicillin-resistant Staphylococcus aureus (MRSA). One 1200-mg dose has been demonstrated to be noninferior to 7 to 10 days of twice-daily IV vancomycin in 2 randomized controlled Phase 3 trials assessing safety and efficacy [6, 7]. Due to its single-dose treatment and no requirement for drug therapeutic monitoring, oritavancin may be an ideal treatment in the ambulatory setting in selected patients. Given the clinical need for meaningful comparative efficacy data on ABSSSI therapies in the outpatient setting, this study compared the efficacy and safety of oritavancin and vancomycin among a subgroup of patients in the Phase 3 SOLO clinical trials who received their entire course of therapy in the outpatient setting.
METHODS
Study Design and Patient Population
The design and overall results of the SOLO I and SOLO II clinical trials are described in detail elsewhere [6, 7]. In brief, these were 2 identical, Phase 3, multicountry, multicenter, randomized, double-blind, noninferiority, and comparative efficacy and safety studies that evaluated a single 1200-mg dose of IV oritavancin compared with twice-daily IV vancomycin (1 gram or 15 mg/kg), for 7 to 10 days in adults with ABSSSI caused, or suspected to be caused, by Gram-positive pathogens.
The SOLO I and SOLO II protocols were originally designed so that patients were hospitalized until assessments of early clinical evaluation (ECE) were completed at 48 to 72 hours after the initiation of treatment (primary endpoint). The protocols were amended (Amendment 2) 5 months into enrollment to allow US patients to complete their entire course of antimicrobial therapy in an outpatient setting at the discretion of the investigator. The studies were conducted in accordance with the 2008 Declaration of Helsinki. Institutional review board or ethics committee approval was obtained at each participating center. All participants provided written informed consent (Trial Registration: https://clinicaltrials.gov/ct2/show/NCT01252719?term=oritavancin&rank=6, https://clinicaltrials.gov/ct2/show/NCT01252732?term=oritavancin&rank=7).
Per the protocol, acquisition of an ABSSSI site specimen for Gram stain, culture, and susceptibility testing was collected for all patients within 24 hours before initiation of study drug. For abscesses or wound infections, biopsy, needle aspiration, deep swab, or surgically obtained specimens were the preferred methods. For cellulitis patients, punch biopsy of the leading edge of erythema was preferred, but needle aspiration was also permitted.
Clinical Outcome Measures
The primary efficacy endpoint was a composite clinical outcome at ECE at 48 to 72 hours that comprised (1) cessation of spreading or reduction in the size of the baseline lesion, (2) absence of fever, and (3) no rescue antibiotic medication. The key secondary endpoints were investigator-assessed clinical cure at posttherapy evaluation (PTE) and reduction in size of baseline lesion ≥20% at ECE. Exploratory treatment-outcomes analyses were conducted in a variety of subgroups including age, sex, race, weight, body mass index, region, presence of diabetes mellitus, hepatic impairment, immunodeficiency, infection type, baseline renal function (creatinine clearance [CLCR], presence of S aureus [MRSA vs methicillin-sensitive S aureus vs none]), presence of systemic inflammatory response syndrome criteria, receipt of antibiotics before study drug, IV drug use (IVDU), presence of bacteremia, and fever at baseline.
Safety Assessments
Safety was monitored for an extended follow-up period of 60 days. Safety assessments included vital signs, electrocardiograms, clinical chemistry and hematologic assessments, rates of adverse events (AEs), and rates of serious AEs (SAEs). Treatment-emergent AEs (TEAEs) consisted of AEs that began or worsened in severity at the time of or following the first dose of study drug. As part of safety assessments, frequency and type of TEAEs, time to onset, and duration of (1) all TEAEs and drug-related TEAEs, (2) hypersensitivity and infusion reactions, and (3) infusion site reaction/phlebitis were recorded. Post hoc analyses of occurrences of nephrotoxicity, defined as either a 50% or 0.5 mg/dL increase in serum creatinine, whichever was greater, from initiation of vancomycin to 48 hours postcompletion, and hospital admissions from start of antimicrobial treatment in the outpatient setting to PTE were also performed [8, 9].
Statistical Methods
All outcome and safety analyses presented were performed on patients who received antimicrobial treatment in an outpatient setting, also known as the outpatient subgroup, in a post hoc analysis. The outpatient subgroup in the current analysis is defined as US patients randomized after Amendment 2, who received antimicrobial treatment exclusively in the outpatient setting. Outcome event rates and 2-sided 95% confidence intervals (CIs) of the difference between event rates for oritavancin and vancomycin were presented based on the outpatient subgroup of the modified intention-to-treat population. Modified intention-to-treat population included all patients who underwent randomization and received either oritavancin or vancomycin. The frequency and severity of TEAEs were presented for both treatment arms using descriptive statistics. Adverse events were recorded regardless of relationship to study drug, coded by Medical Dictionary for Drug Regulatory Activities (MedDRA, version 13.1), and summarized by frequency, severity, relationship to study drug as assessed by the investigator, onset, and duration using the primary system organ class and preferred term.
RESULTS
Outpatient Patient Population and Demographics/Baseline Characteristics
The SOLO studies comprised 1987 randomized patients. The outpatient subgroup described in the current analysis consisted of 792 patients treated in the outpatient setting exclusively (392 in the oritavancin arm and 400 in the vancomycin arm). The outpatient subgroup accounted for 74% of patients enrolled in the United States after Amendment 2 (Figure S1). The majority (74%) of patients in the outpatient subgroup received oritavancin or vancomycin in a nonemergency department outpatient clinic (Table S1). The mean daily vancomycin dose was 2.5 grams, and mean duration of vancomycin therapy was 7.5 days. Vancomycin trough levels were assessed for 366 of 400 vancomycin patients; mean trough level was 12.3 mg/L. Investigator-assessed clinical cure and failure rates at PTE were similar among patients whose initial trough value was ≤10 mg/L, >10–15 mg/L, and >15 mg/L (Table S2).
Demographics and baseline characteristics for the patients in the outpatient subgroup were similar between oritavancin and vancomycin treatment groups (Table 1). The mean age of patients was 42 years, and most patients were white and male. Infection types were balanced in the oritavancin and vancomycin groups, with approximately 30% cellulitis, 38% abscess, and 32% wound infection. A Gram-positive pathogen known to cause ABSSSI was isolated from 624 patients (79%) at baseline. Staphylococcus aureus was the most common (74%), and MRSA was confirmed in 254 of 461 S aureus patients (55%). The median infection area at baseline was 252.0 cm2 for the oritavancin group and 257.8 cm2 for the vancomycin group. A significant proportion of the outpatient subgroup reported IV drug use (55%), or patients were diagnosed with hepatitis or other hepatic condition (33%) or diabetes mellitus (9%).
Table 1.
Demographic and Baseline Characteristics in the Outpatient Subgroup of mITT Population
| Characteristic | Oritavancin (N = 392) | Vancomycin (N = 400) |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 42.2 (12.14) | 41.6 (12.38) |
| Median (min, max) | 43.0 (18, 80) | 42.0 (18, 79) |
| ≥65 years (n [%]) | 16 (4.1) | 8 (2.0) |
| Gender (n [%]) | ||
| Male | 241 (61.5) | 254 (63.5) |
| Race (n [%]) | ||
| White | 341 (87.0) | 351 (87.8) |
| Black | 35 (8.9) | 31 (7.8) |
| Asian | 3 (0.8) | 5 (1.3) |
| Other | 13 (3.3) | 13 (3.3) |
| Body weight (kg) | ||
| Mean (SD) | 83.0 (21.59) | 86.0 (23.93) |
| Median (min, max) | 79.5 (43, 200) | 81.6 (41, 189) |
| Body Mass Index (kg/m2) | ||
| Mean (SD) | 28.5 (7.62) | 29.1 (7.94) |
| Median (min, max) | 26.9 (16, 67) | 27.0 (16, 65) |
| <25 kg/m2 (n [%]) | 138 (35.2) | 140 (35.0) |
| 25 to <30 kg/m2 (n [%]) | 126 (32.1) | 124 (31.0) |
| ≥30 kg/m2 (n [%]) | 128 (32.7) | 136 (34.0) |
| Diabetes mellitus | 32 (8.2%) | 37 (9.3%) |
| Hepatitis, or other hepatic condition | 130 (33.2%) | 133 (33.3%) |
| Acquired immunodeficiency syndrome, or other immune deficiency state | 4 (1.0%) | 6 (1.5%) |
| Infection Type (n [%]) | ||
| Wound infection | 128 (32.7) | 125 (31.3) |
| Cellulitis | 112 (28.6) | 122 (30.5) |
| Major cutaneous abscess | 152 (38.8) | 153 (38.3) |
| Lesion Area (cm2) | ||
| Mean (SD) | 369.4 (341.57) | 400.7 (418.21) |
| Median (min, max) | 252.0 (75.0, 2310.0) | 257.8 (77.0, 3417.0) |
| Renal insufficiency or renal failure (based on medical history) |
6 (1.5%) | 4 (1.0%) |
| Confirmed pathogen at baseline | 364/392 (93%) | 365/400 (91%) |
| Staphylococcus aureus | 227/364 (62.4%) | 234/365 (64.1%) |
| MSSA at baseline (n) | 98/364 (26.9%) | 109/365 (29.9%) |
| MRSA at baseline (n) | 129/364 (35.4%) | 125/365 (34.2%) |
| Meeting SIRS criteria (n [%]) | 34 (8.7) | 38 (9.5) |
| IV drug use | 218 (55.6%) | 218 (54.5%) |
| Temperature ≥38.0°C (proportion [%]) | 17/392 (4.3) | 16/399 (4.0) |
| Severe peripheral vascular conditions | 4 (1.0%) | 1 (0.3%) |
Abbreviations: IV, intraveneous; max, maximum; min, minimum; mITT, modified intention-to-treat population; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive S aureus; SD, standard deviation; SIRS, systemic inflammatory response syndrome.
Primary and Secondary Efficacy Endpoints in Outpatients
Response rates for the primary composite endpoint at ECE were comparable between treatment groups (80.4% vs 77.5% [difference = 2.9%; 95% CI, −2.8 to 8.5] for oritavancin and vancomycin, respectively). The proportion of patients who achieved a lesion size reduction ≥20% at ECE was 87.0% in the oritavancin group vs 83.8% in the vancomycin group (difference = 3.2%; 95% CI, −1.7 to 8.2) (Table 2). The investigator-assessed clinical cure rates at PTE were 83.4% and 80.5% (difference = 2.9%; 95% CI, −2.4 to 8.3) for oritavancin and vancomycin, respectively. The response rates for the primary and secondary efficacy outcomes were similar between the oritavancin and vancomycin groups across most subgroups with a few exceptions (Figure 1A–C). The primary composite efficacy response rates at ECE were significantly different between treatment groups among patients ≥100 kg (85.7% oritavancin vs 73.2% vancomycin; difference = 12.5%; 95% CI, 2.3–26.6). More patients in the MRSA subgroup had a ≥20% lesion size reduction at ECE in the oritavancin treatment group versus the vancomycin treatment group (93.0% vs 84.8%; difference = 9.1%; 95% CI, 2.4–15.8). Investigator-assessed clinical cure rates at PTE were higher in the oritavancin treatment group relative to the vancomycin treatment group for patients with a wound infection (85.9% vs 74.4%; difference = 11.5%; 95% CI, 1.7– 21.2). Differences in efficacy rates between treatment groups by baseline renal function were also observed. Among patients with CLCR ≥90 mL/min, higher primary composite response rates at ECE were observed in the oritavancin group relative to the vancomycin group (82.6% vs 75.3%; difference = 7.4%; 95% CI, 1.3–13.5). Among patients with a CLCR between 60 and 90 mL/min, primary composite efficacy response rates at ECE were lower among patients who received oritavancin versus vancomycin (67.4% vs 91.3%; difference = −20.9%; 95% CI, −35.9 to −5.9).
Table 2.
Primary and Secondary Endpoints Among 792 Patients Treated in the Outpatient Setting (mITT Population)
| Endpoint/Population | Oritavancin (N = 392) n/N (%) |
Vancomycin (N = 400) n/N (%) |
Difference (95% CI) |
|---|---|---|---|
| Primary composite endpoint at ECE | 315 (80.4%) | 310 (77.5%) | 2.9 (−2.8 to 8.5) |
| ≥20% lesion size reduction at ECE | 341 (87.0%) | 335 (83.8%) | 3.2 (−1.7 to 8.2) |
| Investigator-assessed clinical cure at PTE | 327 (83.4%) | 322 (80.5%) | 2.9 (−2.4 to 8.3) |
| Sustained clinical response at PTE | 286 (73.0%) | 282 (70.5%) | 2.5 (−3.7 to 8.8) |
Abbreviations: CI, confidence interval; ECE, early clinical evaluation; mITT, modified intention-to-treat population; PTE, posttherapy evaluation.
Figure 1.
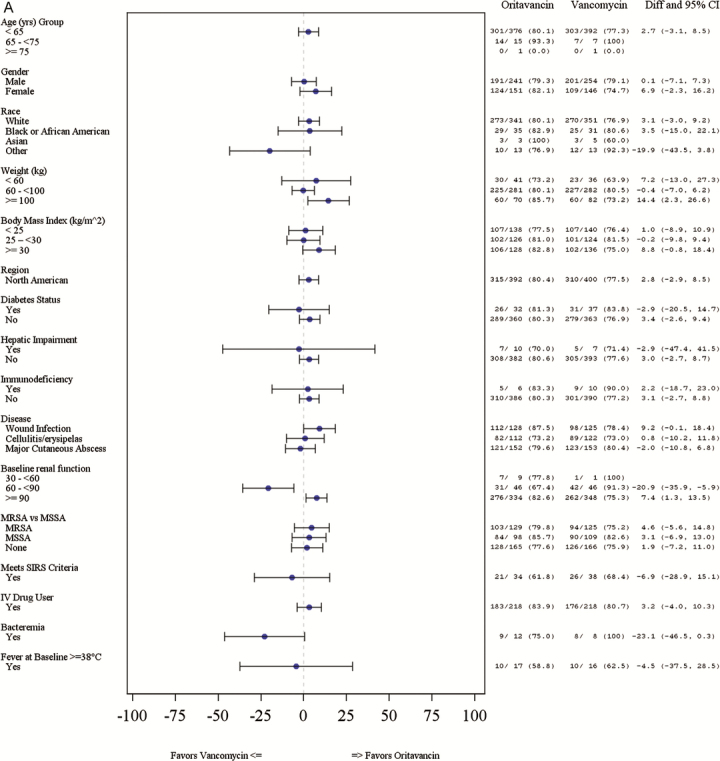
(A) Primary efficacy outcomes at early clinical evaluation (ECE) by subgroup*. *Whisker plots not available where results are 0% or 100%. CI, confidence interval; Diff, difference; IV, intravenous; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive S aureus; SIRS, systemic inflammatory response syndrome.
Figure 1.
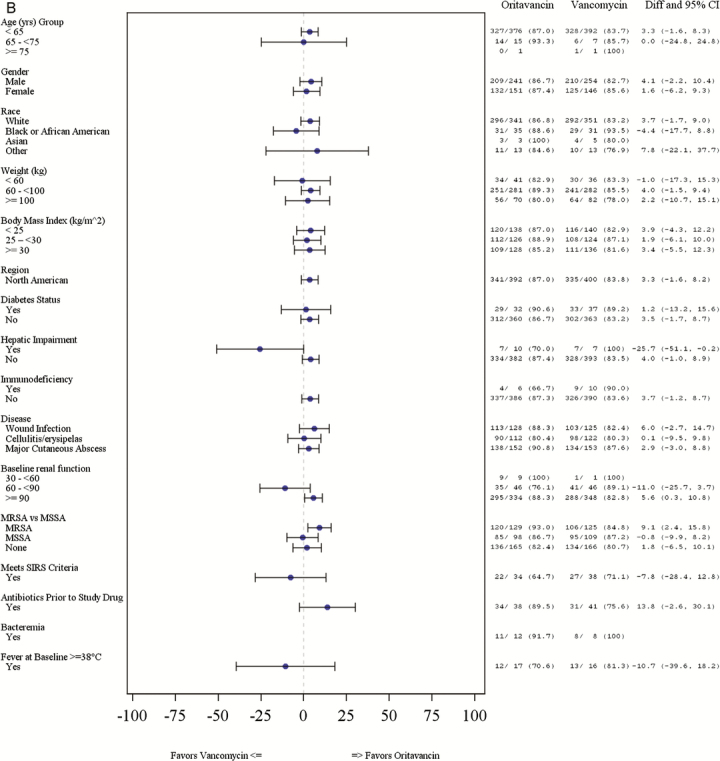
(B) Patients with ≥20% lesion size reduction from baseline at ECE by subgroup. *Whisker plots not available where results are 0% or 100%. CI, confidence interval; Diff, difference; IV, intravenous; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive S aureus; SIRS, systemic inflammatory response syndrome.
Figure 1.
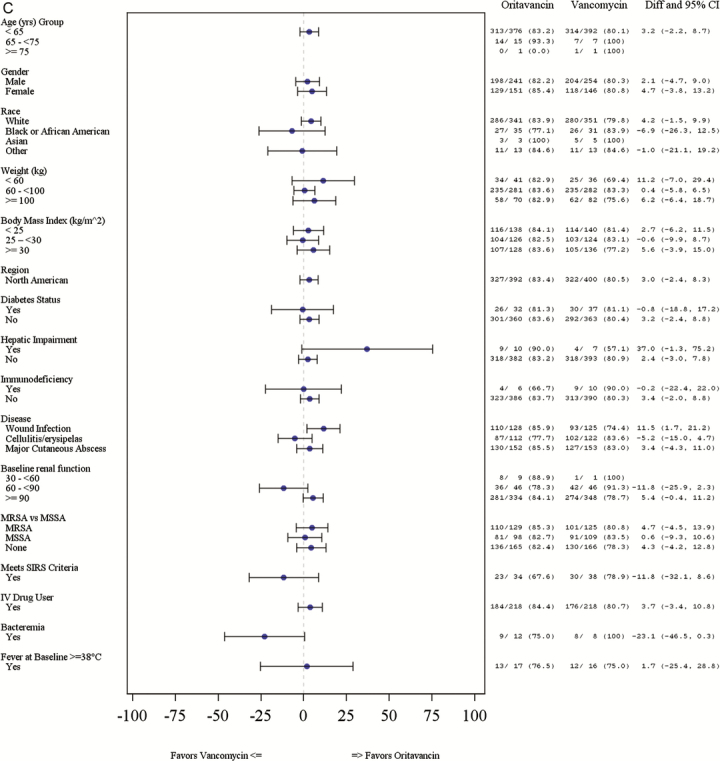
(C) Investigator-assessed clinical cure at posttherapy evaluation by subgroup *. *Whisker plots not available where results are 0% or 100%. CI, confidence interval; Diff, difference; IV, intravenous; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive S aureus; SIRS, systemic inflammatory response syndrome.
Safety Outcomes in Outpatients
The incidence of TEAEs was similar between treatment groups; 65.8% of oritavancin patients experienced at least 1 TEAE compared with 69.0% of vancomycin patients (Table 3). The number of patients who experienced a drug-related AE, as assessed by the investigator, was lower in the oritavancin group compared with the vancomycin group (32.7% vs 43.3%). Rates of blinded oritavancin/placebo or vancomycin discontinuation due to AEs were similar between the 2 groups (4.8% vs 5.8%). Serious AEs were comparable between treatment groups (6.1% of oritavancin patients vs 6.5% of vancomycin patients). There were no cases of osteomyelitis amongst either oritavancin- or vancomycin-treated outpatients. No drug-related AEs or SAEs were fatal. Time to onset of all TEAEs was identical between treatment groups (median of 3.0 days), whereas median duration of AEs was longer for the oritavancin group than the vancomycin group (3.0 days vs 2.0 days).
Table 3.
Overall Summary of Treatment-Emergent Adverse Events Among Patients Treated in the Outpatient Setting (Safety Population)a
| Parameter | Oritavancin (N = 392) n (%) |
Vancomycin (N = 400) n (%) |
Total (N = 792) n (%) |
|---|---|---|---|
| At least one TEAE | 258 (65.8%) | 276 (69.0%) | 534 (67.4%) |
| Study drug-relatedb TEAE | 128 (32.7%) | 173 (43.3%) | 301 (38.0%) |
| TEAE leading to study drug discontinuationc | 19 (4.8%) | 23 (5.8%) | 42 (5.3%) |
| SAE | 24 (6.1%) | 26 (6.5%) | 50 (6.3%) |
| Death | 0 | 0 | 0 |
| Most Commonly Reported TEAEs (≥2% in Oritavancin Group) | |||
| Nausea | 61 (15.6%) | 66 (16.5%) | 127 (16.0%) |
| Hypersensitivity | 35 (8.9%) | 91 (22.8%) | 126 (15.9%) |
| Headache | 35 (8.9%) | 27 (6.8%) | 62 (7.8%) |
| Vomiting | 30 (7.7%) | 31 (7.8%) | 61 (7.7%) |
| Cellulitis | 24 (6.1%) | 18 (4.5%) | 42 (5.3%) |
| Diarrhea | 23 (5.9%) | 21 (5.3%) | 44 (5.6%) |
| Abscess limb | 20 (5.1%) | 12 (3.0%) | 32 (4.0%) |
| Tachycardia | 19 (4.8%) | 8 (2.0%) | 27 (3.4%) |
| Pruritus | 13 (3.3%) | 56 (14.0%) | 69 (8.7%) |
| Dizziness | 12 (3.1%) | 14 (3.5%) | 26 (3.3%) |
| Abscess | 10 (2.6%) | 3 (0.8%) | 13 (1.6%) |
| Fatigue | 10 (2.6%) | 7 (1.8%) | 17 (2.1%) |
| Infection | 10 (2.6%) | 1 (0.3%) | 11 (1.4%) |
| Infusion site phlebitis | 10 (2.6%) | 5 (1.3%) | 15 (1.9%) |
| Subcutaneous abscess | 9 (2.3%) | 5 (1.3%) | 14 (1.8%) |
| Constipation | 8 (2.0%) | 18 (4.5%) | 26 (3.3%) |
| Infusion site extravasation | 15 (3.8%) | 8 (2.0%) | 23 (2.9%) |
Abbreviations: AEs, adverse events; MedDRA, Medical Dictionary for Drug Regulatory Activities; TEAE, treatment-emergent adverse event; SAE, serious adverse event.
TEAEs are adverse events that occurred or whose severities worsened on or after the initiation of study drug. Patients with multiple AEs are only counted once within each MedDRA level.
Includes adverse events considered by the investigators as definitely related or possibly related to the study drug.
Because oritavancin was given as a single dose in a blinded fashion, “study drug discontinuation” means discontinuation of twice-daily placebo infusions.
The most frequently reported TEAEs (≥2% of patients) were similar between the treatment groups (Table 3). Rates of pruritus were lower in the oritavancin group relative to the vancomycin group (3.3% vs 14.0%). The incidence of hypersensitivity was less frequently reported among oritavancin patients compared with vancomycin patients (8.9% vs 22.8%). The median time to hypersensitivity onset was 1 day, and hypersensitivity duration was 3 days for oritavancin group compared with a median time to onset of 1 day and duration of 1 day for vancomycin. Rates of cellulitis (6.1% vs 4.5%), abscess limb (5.1% vs 3.0%), and tachycardia (4.8% vs 2.0%) were higher in the oritavancin arm relative to vancomycin.
Of the 392 patients in the oritavancin treatment group, 5 (1.3%) were admitted to a hospital posttreatment, compared with 9 of 400 patients (2.3%) in the vancomycin group. In the 5 oritavancin patients, hospitalizations were related to worsening or progression of index infection and occurred between 3 to 10 days of treatment initiation. In the 9 vancomycin patients, hospitalizations were related to worsening, progression, or nonimprovement of index infection and occurred between 2 to 16 days of treatment initiation.
DISCUSSION
Historically, enrollment for ABSSSI trials has predominately occurred in the inpatient setting. Although these trials have provided invaluable information on the efficacy and safety of new agents, outcomes may be dependent on the setting of care. Cognizant of these issues, this analysis was designed to compare efficacy and safety outcomes in patients who were randomized to receive either a single dose of oritavancin or 7–10 days of twice-daily vancomycin exclusively in the outpatient setting in the Phase 3 SOLO clinical trials.
Overall, approximately 800 US patients received the entire course of oritavancin or vancomycin in the outpatient setting in the SOLO trials post Amendment 2. As one would expect in clinical trials of this size, treatment groups were highly similar at baseline and similar to the overall SOLO population. The median lesion size was in excess of 250 cm2, and most patients had associated comorbidities, comparable with other ABSSSI trials where patients were treated entirely in the inpatient setting [10–13]. Patients with ABSSSI due to S aureus were common in both treatment groups. A total of 254 patients had MRSA at baseline, or 32% of the total patient population in the outpatient subgroup. This constitutes the largest set of MRSA-infected patients studied since revised US Food and Drug Administration guidance was published.
There were a few notable differences between patients managed in the outpatient setting relative to the inpatient setting in the SOLO trials. Compared with the overall SOLO population, the outpatient subgroup was slightly younger, included a higher proportion of white race, more IVDUs, and higher incidence of abscess than the overall SOLO population [6, 7]. Because treatment setting was at the discretion of the investigator, these differences likely reflect physician assessment about which patients may do well in the outpatient setting.
With regards to efficacy, the clinical response rates at ECE and investigator-assessed clinical cure rates at PTE were similar between groups and across various subgroups. As expected, there were a few numerical differences noted between treatment groups in the subgroup efficacy analyses, and most were in favor of oritavancin. It is important to note that the study was neither designed nor powered to evaluate efficacy across these subgroups, and caution should be exercised when interpreting these findings. However, the overall positive efficacy results and subgroup analyses findings with oritavancin relative to vancomycin in this diverse ABSSSI study population reflect the potential ability of oritavancin to shift the site of care from the inpatient to the ambulatory setting for a large portion of patients that present with ABSSSI.
The safety and tolerability profile of oritavancin was also found to be similar to vancomycin in the outpatient setting. Consistent with most Phase 3 ABSSSI trials, a fair percentage of patients had TEAEs and study-related TEAEs. Nausea, headache, and vomiting were the most frequently reported TEAEs with oritavancin. Rates of pruritus and hypersensitivity reactions, 2 important safety and tolerability considerations with use of glycopeptide antibiotics, were considerably lower in the oritavancin group relative to the vancomycin group, whereas rates of cellulitis, abscess limb, and tachycardia were higher. Of note, although cellulitis and abscess reflect the condition being treated, they were also reported as AEs by investigators and therefore included in this analysis for the purpose of transparency. Time to onset of AEs was similar, but duration of AEs was longer in the oritavancin group, a finding that should be explored further. More importantly, less than 5% of oritavancin patients had a TEAE that resulted in study drug/placebo discontinuation, and only 1.3% of patients in the oritavancin group were subsequently admitted to a hospital compared with 2.3% of vancomycin patients.
Although there are challenges to directly extrapolating real-world effectiveness outcomes from Phase 3 ABSSSI trials, there does not appear to be any major safety or tolerability concerns with use of a single dose of oritavancin in the outpatient setting. The rates of hospitalization observed in this trial with oritavancin are also considerably lower than the 30-day readmission rates noted for most institutions among hospitalized patients with a primary admissions diagnosis for a skin infection [14, 15]. This is an important consideration for use given the increasing adoption of this quality metric across US healthcare inpatient facilitates.
Several things should be noted when interpreting these findings. The outpatient subgroup from the SOLO trials included only patients in the United States. Although we do not anticipate different findings across different countries, the external validity of the study findings need to be confirmed before they can be applied to patients from other countries, especially those with different capacities for outpatient care for patients with ABSSSI. In addition, this was a randomized, multicenter, double-blind study designed to assess efficacy. To maintain blinding, patients in the oritivancin group were required to return to the outpatient setting twice daily to receive placebo doses, which provided additional follow-up with a healthcare provider and may have inflated the rate of infusion-related reactions and AEs relative to what would be expected with a single-dose in the real world. The real-world implications of a single dose administered in the outpatient setting must be explored, specifically outcomes of patients who do not have daily follow-up visits as well as the potential clinical benefit of guaranteed adherence that single dosing provides. Lastly, only 20% of patients have an initial trough value >15 mg/L (Table S2), a proportion lower than that reported with nomogram dosing of vancomycin in the inpatient setting [16]. It is important to note that the vancomycin consensus statement does not provide definitive targeted trough recommendations for patients with ABSSSIs [17]. More importantly, investigator-assessed clinical cure and failure rates at PTE were similar among patients whose initial trough value was ≤10 mg/L, >10–15 mg/L, and >15 mg/L (Table S2). In addition, the trough values observed in this study are likely reflective of the difficulty of carefully maintaining trough levels in a narrow targeted range in patients treated in an outpatient setting. The intensive therapeutic monitoring that is possible in the inpatient setting may not be viable in the outpatient setting.
CONCLUSIONS
In conclusion, this post hoc analysis shows that a single dose of oritivancin has comparable efficacy outcomes to twice-daily IV vancomycin for 7–10 days in patients who were treated in the outpatient setting. Oritavancin was generally well tolerated, with lower incidences of hypersensitivity reactions and pruritus relative to vancomycin. In addition, very few patients in the oritavancin group required subsequent care in the inpatient setting post-outpatient treatment. Given current US healthcare expenditures for ABSSSI treatment in the inpatient setting, a single dose of oritavancin in the ambulatory setting may represent a treatment option for patients with ABSSSI that can safely and effectively shift care from the inpatient to the outpatient setting, while minimizing outpatient healthcare resource use.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
Author contributions. The Medicines Company designed and conducted the study. The Medicines Company, in collaboration with the authors, analyzed and interpreted the data.
Financial support. This work was supported by The Medicines Company.
Potential conflicts of interest. T. P. L. is a paid consultant and speaker for The Medicines Company (Parsippany, NJ); G. R. C. is a paid consultant and advisor to The Medicines Company (Parsippany, NJ); M. R., S. O. A., and K. A. S. are employees and shareholders of The Medicines Company (Parsippany, NJ).
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Agency for Healthcare Research and Quality, 2011 National Statistics: All ED visits. Available at: http://hcupnet.ahrq.gov/HCUPnet.jsp?Parms=H4sIAAAA AA AAAEuxTPNzdQk2NEzNyzZMS0sMTkpNynGJCAhKA4PUzLTEQleX sKTMpGIgLgGJAQAP0Gj9MwAAAAF9F36C42E3DB7294FD516624A A628A24D0ABF881&JS=Y Accessed 15 May 2016
- 2. Edelsberg J, Taneja C, Zervos M, et al. Trends in US hospital admissions for skin and soft tissue infections. Emerg Infect Dis 2009; 15:1516–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lodise TP, Fan W, Sulham KA. Economic impact of oritavancin for the treatment of acute bacterial skin and skin structure infections in the emergency department or observation setting: cost savings associated with avoidable hospitalizations. Clin Ther 2016; 38:136–48. [DOI] [PubMed] [Google Scholar]
- 4. Lodise TP, Fan W, Sulham KA. Hospital admission patterns in adult patients with skin and soft tissue infections: identification of potentially avoidable hospital admissions through a retrospective database analysis. Hosp Pract (1995) 2015; 43:137–43. [DOI] [PubMed] [Google Scholar]
- 5. Talan DA, Salhi BA, Moran GJ, et al. Factors associated with decision to hospitalize emergency department patients with skin and soft tissue infection. West J Emerg Med 2015; 16:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corey GR, Kabler H, Mehra P, et al. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med 2014; 370:2180–90. [DOI] [PubMed] [Google Scholar]
- 7. Corey GR, Good S, Jiang H, et al. Single-dose oritavancin versus 7–10 days of vancomycin in the treatment of Gram-positive acute bacterial skin and skin structure infections: the SOLO II noninferiority study. Clin Infect Dis 2015; 60:254–62. [DOI] [PubMed] [Google Scholar]
- 8. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009; 66:82–98. [DOI] [PubMed] [Google Scholar]
- 9. Lodise TP, Patel N, Lomaestro BM, et al. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis 2009; 49:507–14. [DOI] [PubMed] [Google Scholar]
- 10. Arbeit RD, Maki D, Tally FP, et al. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin Infect Dis 2004; 38:1673–81. [DOI] [PubMed] [Google Scholar]
- 11. Friedland HD, O’Neal T, Biek D, et al. CANVAS 1 and 2: analysis of clinical response at day 3 in two phase 3 trials of ceftaroline fosamil versus vancomycin plus aztreonam in treatment of acute bacterial skin and skin structure infections. Antimicrob Agents Chemother 2012; 56:2231–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prokocimer P, De Anda C, Fang E, et al. Tedizolid phosphate vs linezolid for treatment of acute bacterial skin and skin structure infections: the ESTABLISH-1 randomized trial. JAMA 2013; 309:559–69. [DOI] [PubMed] [Google Scholar]
- 13. Boucher HW, Wilcox M, Talbot GH, et al. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med 2014; 370:2169–79. [DOI] [PubMed] [Google Scholar]
- 14. Martin ET, Evans R, Comptom M, et al. All-cause readmissions following treatment with linezolid for MRSA-ABSSSI: a propensity score analysis [poster]. ID Week 2015 (San Diego, CA). [Google Scholar]
- 15. Pasquale TR, Trienski TL, Olexia DE, et al. Evaluation of the impact of an antimicrobial stewardship program in patients with acute bacterial skin and skin structure infections (ABSSSI) at a teaching hospital [poster number 744]. ID Week 2012 (San Diego, CA). [DOI] [PubMed] [Google Scholar]
- 16. Kullar R, Leonard SN, Davis SL, et al. Validation of the effectiveness of a vancomycin nomogram in achieving target trough concentrations of 15–20 mg/L suggested by the vancomycin consensus guidelines. Pharmacotherapy 2011; 31:441–8. [DOI] [PubMed] [Google Scholar]
- 17. Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis 2009; 49:325–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


