Abstract
Background
Urinary tract infection (UTI) as the most common healthcare-associated infection accounts for up to 36% of all healthcare-associated infections. Catheter-associated urinary tract infection (CAUTI) accounts for up to 80% of these. In many instances indwelling urinary catheter (IDC) insertions may be unjustified or inappropriate, creating potentially avoidable and significant patient distress, embarrassment, discomfort, pain and activity restrictions, together with substantial care burden, costs and hospitalisation. Multifaceted interventions combining best practice guidelines with staff engagement, education and monitoring have been shown to be more effective in bringing about practice change than those that focus on a single intervention. This study builds on a nurse-led initiative that identified that significant benefits could be achieved through a systematic approach to implementation of evidence-based practice.
Methods
The primary aim of the study is to reduce IDC usage rates by reducing inappropriate urinary catheterisation and duration of catheterisation. The study will employ a multiple pre-post control intervention design using a phased mixed method approach. A multifaceted intervention will be implemented and evaluated in four acute care hospitals in NSW, Australia. The study design is novel and strengthened by a phased approach across sites which allows for a built-in control mechanism and also reduces secular effects. Feedback of point prevalence data will be utilised to engage staff and improve compliance. Ward-based champions will help to steward the change and maintain focus.
Discussion
This study will improve patient safety through implementation and robust evaluation of clinical practice and practice change. It is anticipated that it will contribute to a significant improvement in patient experiences and health care outcomes. The provision of baseline data will provide a platform from which to ensure ongoing improvement and normalisation of best practice. This study will add to the evidence base through enhancing understanding of interventions to reduce CAUTI and provides a prototype for other studies focussed on reduction of hospital acquired harms. Study findings will inform undergraduate and continuing education for health professionals.
Trial registration
ACTRN12617000090314. Registered 17 January 2017. Retrospectively registered.
Electronic supplementary material
The online version of this article (doi:10.1186/s12913-017-2268-2) contains supplementary material, which is available to authorized users.
Keywords: Healthcare-associated infection, Catheter-associated urinary tract infection, Multifaceted intervention, Evidence-based practice
Background
Urinary tract infection (UTI) is considered the most common healthcare-associated infection (HAI) [1], accounting for up to 36% of all healthcare-associated infections (HAIs) [2]. Catheter-associated urinary tract infections (CAUTIs) represent the majority of UTIs (up to 67% of UTIs in all hospital inpatients [3], and up to 97% in ICUs [4]). Between 12 and 16% of hospitalised patients may receive a short term indwelling urinary catheter (IDC) [5], and many of these IDC insertions have been identified as unjustified or inappropriate [6]. CAUTI risk increases considerably with duration of catheterisation [7], and generates substantial care burden and significant hospitalisation costs, patient distress, embarrassment, discomfort, pain and activity restrictions [7–9]. A recent Australian study indicated that 1.7% of inpatients, hospitalised for > 48 h, contract a UTI, adding additional days (mean = 4) to their length of stay (LoS) [10].
CAUTI is possibly the most preventable HAI [11], with significant potential cost savings. According to Mitchell et al.’s (2016) [10] calculations, there are ~380,600 extra public hospital bed-days used each year in Australia due to healthcare-associated urinary tract infections (the majority being catheter-associated [3]). Umscheid et al. (2011) [11] estimate that each CAUTI costs between $1200 and $4700 USD. In the Australian setting, Jackson et al. (2011) estimated that the costs associated with a patient diagnosed with CAUTI are twice as much as a patient not affected by CAUTI [12].
Preventing CAUTI
Worldwide, there has been renewed interest and research into reducing the incidence of CAUTI, especially in the USA, with the introduction of non-payment for ‘reasonably preventable’ hospital-acquired complications [13]. An integrative review by Meddings et al. (2014) [1] evaluated interventions up to October 2012 to reduce IDC usage and CAUTIs. Meddings et al. (2014) found that interventions to reduce inappropriate IDC use, and bundles of interventions focusing on reducing unnecessary catheter use and general infection control were successful in reducing catheter use [1]. A component common to most urinary catheter bundles is timely catheter removal [14–20]. Meddings et al. recognised the importance of addressing socioadaptive factors in successfully implementing interventions [1]. These socioadaptive factors have since been addressed in the USA with the Agency for Healthcare Research and Quality (AHRQ) Comprehensive Unit-based Safety Program (CUSP). In a national US study, Saint et al. implemented CUSP in 926 units, and found a significant reduction in catheter use and CAUTI in non-ICUs [19]. Clinician education about recommended practice is a key part of interventions to address catheter use and CAUTI; nine studies since the Meddings et al. 2014 integrative review implemented a hospital (or multi-hospital) intervention to reduce CAUTI, and all included some form of education [14, 17, 19, 21–27]. Indeed, a systematic review of interventions to reduce device-related infections found that all interventions had some form of education as a key component [28].
From evaluating the literature on hospital-wide and multi-hospital interventions designed to reduce urinary catheter use and CAUTI, a gap was identified in study design; all identified studies used a pre-post design, which does not account for secular trends.
Studies investigating use of IDCs and CAUTI have been lacking in the Australian context [29, 30]. Extant literature includes hospital-based rates of IDC usage and/or rates of CAUTI, with some discussion of documentation, appropriate indications for IDC, and staff knowledge. Wynne et al. (2014) [29] found a point prevalence of 12.4% of patients with IDC in situ, in a tertiary teaching hospital in Melbourne. This included an acute inpatient facility and a sub-acute aged care and rehabilitation service. Wynne et al.’s study did not report on the days IDC in situ or prevalence of CAUTI, and a differentiation between short-term and long-term IDC usage was not made. So et al. (2014) conducted a chart audit in a hospital in Sydney, finding catheter utilisation of 11%. A study of staff and patient knowledge of IDC usage in two general medical wards in a Melbourne hospital found that the mean time an IDC was in situ was 5.8 days, and that a physician’s awareness of IDC presence was significantly associated with a shorter time IDC in situ [31]. Giles et al. (2015), in a pilot study, found the prevalence of IDCs in two wards in an Australian hospital (urology ward = 25%; orthopaedic ward = 31%), and rate of CAUTI = 2.2% [32]. Giles et al. then went on to describe the development and pilot of a bundled approach to target IDC utilization and CAUTI, however results of the intervention were not reported. A large point prevalence study in six Australian hospitals by Gardner et al. (2014) found a CAUTI prevalence of 0.9%, and urinary catheter prevalence of 26.3% (88.7% of these being IDCs) [33].
The foregoing studies highlight a gap in knowledge in the Australian healthcare context, in that there have been no studies investigating the effects of an intervention on reducing IDC utilisation and CAUTI rates in an acute care setting.
A multifaceted evidence-based intervention was piloted in two wards in an acute care hospital in the Hunter New England Local Health District, leading to a 50% reduction in IDC insertions, significantly reduced IDC duration and number of patients treated for CAUTI [34].
Building from the positive results from the aforementioned pilot, the present study aims to implement and evaluate an intervention across four acute care hospitals in NSW, Australia. To control for secular trends, implementation of the intervention will be phased across the hospitals.
Methods/Design
Aims
The primary aim of the study is to reduce IDC usage rates by reducing inappropriate urinary catheterisation and duration of catheterisation.
The secondary aims of the study are to identify:
The current inpatient indwelling urinary catheter usage rate and incidence of CAUTI;
If the implementation and adherence to bundled catheter care (BCC) reduces IDC use and CAUTI;
How effective BCC is in improving IDC-related outcomes;
The barriers and enablers to successful implementation of BCC; and
The cost benefits of implementation.
Design
The study will employ a multiple pre-post control intervention design using a phased mixed method approach (Fig. 1).
Fig. 1.
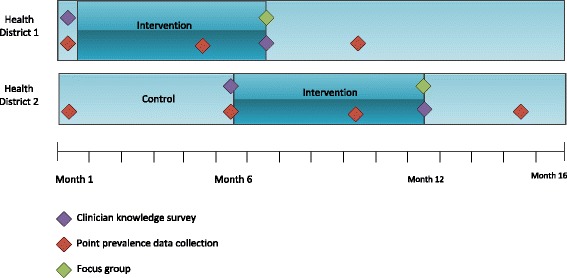
Study Design. Data collection points are indicated with diamonds on timeline
Implementation of the intervention across four acute care hospitals will be staged, with multiple clusters in each of two implementation stages. Pre and post point prevalence data comparison will occur within all hospitals pre and post intervention, as well as between the two Health Districts as detailed in Fig. 1.
The staged implementation of the intervention allows for a control between the two Health Districts.
A mixed method design provides a platform to explore in-depth existing barriers and enablers related to implementing practice change. The sequential phased nature of the study ensures that the necessary evidence is available to inform the subsequent implementation phase of the study. The focus groups will identify barriers and enablers to implementation and uptake and will inform strategies to embed the intervention into normal practice. Questions in the focus groups will be informed by results from the point prevalence and clinician surveys.
The control will be usual urinary catheterisation practice, i.e., no intervention or implementation strategies. NSW Health evidence-based practice guidelines for “Adult Urethral Catheterisation for Acute Care Settings” [35], and local clinical practice guidelines for urinary catheterisation for each Health District are available for all clinicians, and can be accessed online.
The Clinical Excellence Commission (CEC), a corporation addressing patient safety and clinical quality in the NSW Health context, established a CAUTI project in 2014 to “help healthcare professionals in reducing the incidence of CAUTIs in acute care settings” [36]. A urinary catheterisation course on an online NSW Health learning platform also exists [37].
Setting
The intervention sites are four acute care hospitals from two Health Districts in NSW, Australia. Hospitals have been purposively selected, matched on total bed numbers, activity type and activity levels (See Table 1).
Table 1.
Setting
| Health District | Facility | Beds |
|---|---|---|
| 1 | Hospital A | 360 |
| Hospital B | 260 | |
| Total beds Health District 1 = 620 | ||
| 2 | Hospital C | 549 |
| Hospital D | 318 | |
| Total beds Health District 2 = 867 | ||
A key difference between the two health districts is the system used for medical health records: Health District 1 uses paper-based medical records, whereas Health District 2 uses electronic medical records. Data collection methods and training have been individualised to accommodate these differences.
Data collection
Three main types of data will be collected:
Pre and post implementation point prevalence and patient demographics (quantitative)
Pre and post implementation clinician knowledge and competence (quantitative)
Post implementation perceived barriers and enablers to implementation (qualitative)
Data collection types and details are outlined in Table 2, and the data collection timepoints are displayed in Fig. 1.
Table 2.
Data collection sources and methods
| Data | Data collection method | Data source(s) | Data collected | Data collection timepoint(s) |
|---|---|---|---|---|
| IDC usage rate and incidence of CAUTI | Online data collection tool | - Patient medical records – facility-wide across all four hospitals - Bedside observation - Infection control database |
- Urinary catheter presence - Days catheter in situ - CAUTI rate |
- Baseline - 4 months post-implementation commencement - 9 months post-implementation commencement |
| Patient profile | Data extraction and then merge with data from point prevalence | - Electronic patient management systems | - Patient demographics including age, gender, weight, diagnosis, type of admission | - Baseline - 4 months post-implementation commencement - 9 months post-implementation commencement |
| Clinician knowledge and competency | Online survey | - Clinicians (all nurses and medical officers invited from participating hospitals) | - Clinician competency - Clinician knowledge of CAUTI prevention - Perception of unit-based culture |
- Baseline - 6 months post-implementation commencement |
| Barriers and enablers to implementation | Focus group | - Clinicians (6–8 per facility) (all nurses and medical officers invited from participating hospitals) | - Perceived barriers and enablers to implementation | - 6 months post-implementation commencement |
The point prevalence data will be collected by project staff (clinical nurse consultants, research assistant), nurse and midwife clinicians and clinical nurse educators from each hospital. Training will be administered to all clinicians involved on data collection techniques and definitions prior to collection, and they will be paired where possible with members of the research team. Data collection staff will go to every inpatient bed on every adult inpatient ward in the hospital and input data into a firewall protected online survey tool. Survey data will then be exported and merged with other electronically extracted demographic patient data into statistical package STATA [38] for analysis.
Exclusion and inclusion criteria
Point prevalence data will be collected from all adult inpatient wards across four hospitals in two Health Districts (excluding emergency departments, operating theatres and day only wards).
Multifaceted intervention
The intervention will be delivered in all adult inpatient wards, emergency departments, and operating theatres in all four hospitals. The key component of the intervention is the evidence-based “No CAUTI” bundle (Table 3). To support implementation of the No CAUTI bundle, the following resources were developed as part of the intervention:
IDC insertion criteria guidelines
Indications for IDC specimen collection
Nurse-led IDC removal guidelines (Additional file 1)
Educational resources and compliance auditing tools
Table 3.
Evidence base for No CAUTI Bundle
| N | NEED for catheter assessed – refer to indications, scan bladder, consider alternative, document indication. - Need for IUC is assessed - appropriate indications for insertion [7, 47, 48]. - Scan the bladder to determine bladder volume [7] - Consider alternatives such as external sheath (males),intermittent catheterisation by staff/patient, SPC, double voiding, commode, timed toileting [7, 47, 48] |
| O | OBTAIN patient consent, OFFER patient education including hygiene. - Obtain patient consent and importance of accurate complete documentation. - Provide written and verbal information to patient/carer [49] - Ensure daily meatal hygiene is performed as part of personal hygiene, soap and water is all that is required [7, 47, 48] |
| C | COMPETENCY – clinicians who insert catheters must have documented competency - Competent and trained staff should insert catheters [7, 48] |
| A | ASEPSIS – maintain asepsis & hand hygiene during insertion and while catheter is in place. - Aseptic technique and sterile equipment must be used for IUC insertion. Hand hygiene “Moment 2” and non-sterile gloves is recommended when manipulation of the IUC or drainage system is required. - Empty the bag when ¾ full and use a clean container for each patient; avoid contact between outlet and container. - Maintain a sterile closed system of drainage [7, 48] |
| U | UNOBSTRUCTED flow – no kinks or loops, catheter secured, bag below bladder level and off the floor. - Unobstructed continuous urine flow with no kinks or loops, bag below the bladder and not in touch with any surface. Secure the catheter to the patient to minimise movement and trauma and improve patient comfort [7, 48] |
| T | TIMELY catheter removal and documentation – may be nurse initiated. - Timely removal of the IUC - daily review. Nurse initiated removal guidelines followed if there is no medical documentation for continued use [7, 48] |
| I | INFECTION risk – daily periurethral hygiene. Collect urine specimen only when clinically indicated. - Infection and catheter specimen urine (CSU) collection: must be collected using aseptic technique, from a newly inserted catheter and before the commencement of antimicrobials - CSU should only be collected if clinically indicated [7, 47, 48] |
The distribution and standardised use of a cost-effective, generic IDC insertion pack forms part of the intervention. The insertion pack includes all equipment required for catheterisation, documentation stickers, and securing devices.
Routine assessment of clinician competency in urinary catheter insertion will be introduced as part of the multifaceted intervention.
No CAUTI Bundle
The “bundled intervention” framework used in this project is defined as a collection of a number of evidence based practices or steps, vital to achieving improvement in clinical outcomes [39]. The “No CAUTI” bundle was developed during the pilot project, and is based on evidence-based recommendations. The evidence for the bundled intervention is presented in Table 3.
Implementation strategies
A number of implementation strategies will be used in the project: education, monitoring and feedback, resources, and facilitation. The timing of implementation is displayed in Fig. 2. The Template for Intervention Description and Replication (TIDieR) was developed to improve the quality of descriptions of interventions [40], and can be used to report content of behavior change interventions, including what is delivered, who the intervention is delivered to, and what materials are used. TIDieR has previously been used to describe care bundle interventions [41]. The TIDieR framework has been used to outline the current implementation strategies in Table 4.
Fig. 2.
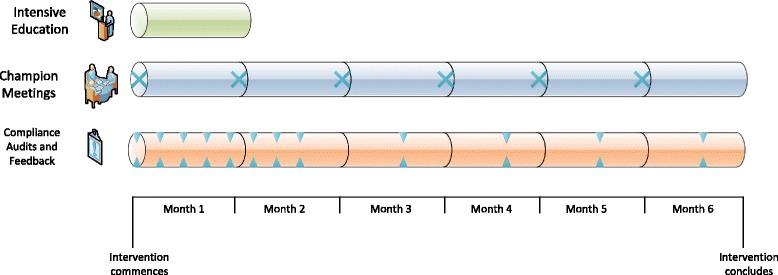
Timeline of implementation components. The intervention commences with four weeks of intensive education. For the first two months, compliance audits are completed on a weekly basis, and then continue on a monthly basis for the remainder of the 6-month intervention period. Champion meetings will be held on a monthly basis throughout the intervention period
Table 4.
“No CAUTI” Implementation strategies summary based on TIDieR
| Implementation Strategy | Rationale | Mode of delivery | Delivered by | Delivered to and where | When/how often |
|---|---|---|---|---|---|
| Education | |||||
| Train-the-trainer workshops | To prepare educators to present the “No CAUTI” bundle to ward-based staff, and to train educators to complete urinary catheterisation competency assessments | Face-to-face (group) | Clinical nurse consultant – urology | Nurse educators from across hospital | 1x 2-3 h workshop at each facility at start of intervention |
| Ward in-services | To familiarise staff with “No CAUTI” bundle and nurse-initiated removal flowchart To identify champions in each ward |
Face-to-face (group) | Nurse educators | Nurses and medical officers from all adult inpatient wards, OTs, and EDs | Minimum 1x 20 min in-service in each ward at start of intervention |
| Monitoring and feedback | |||||
| Compliance audits and feedback | To monitor compliance with “No CAUTI” bundle and provide strategies to support implementation | Individual patient audit, and feedback face-to-face (group) to clinicians | Champions (clinicians previously identified in in-services) | All inpatient wards | Weekly for first two months and then monthly for remaining 4 months of intervention period. |
| Feedback of point prevalence of IDC usage and CAUTI | To focus clinicians on targets and progress | Face-to-face (group) and email | Research project staff | All clinicians at a ward, facility, and district level | Baseline, 4 months, and 9 months |
| Resources | |||||
| “No CAUTI” bundle posters | Prompt awareness and better documentation | Documents displayed in wards | N/A (passive component) | Nurses and medical officers | Ongoing |
| “No CAUTI” bundle badges | Prompt awareness of intervention and identify ward champions | Worn by clinicians and champions | N/A (passive component) | Nurses and medical officers | Ongoing |
| Catheter insertion DVDs | Educate nurses about correct catheterisation processes | Available on intranet | N/A (passive component) | Nurses | Ongoing |
| Facilitation | |||||
| Competency assessments | Increase proportion of clinicians that are competent in urinary catheterisation | Face-to-face (individual) | Nurse educators | Nurses | Ongoing |
| Champions | Act as a resource for clinicians and promote the No CAUTI bundle to clinicians; support implementation | Face-to-face (individual and group) | Nurses | Nurses and medical officers | Ongoing |
Whilst there are key implementation strategies that will be common to all intervention hospitals, there will be a degree of flexibility between the two Health Districts, and their hospitals. Both active (e.g. workshops, audit and feedback) and passive strategies (e.g. distribution and display of posters, equipment) will be used [28].
Previous studies have identified champions as playing a significant role in reinforcing practice change [42]. The need for multiple champions when implementing a large degree of practice change is recommended [43]; the current study will have a champion in each ward, and champions will meet regularly. Nursing staff are critical to the success of bundled interventions aimed at reducing IDC use [27].
Power and sample size calculation
A sample size calculation has indicated that 500 patients per Health District would be sufficient to detect a 40% fall (15 to 9%) in relative IDC insertion rates with a power of 0.8 and alpha 0.05. This is based on a 50% (39.5 to 14.6%) reduction observed in the pilot study [34]. Estimated bed numbers of 860 in Health District 2 and 610 in Health District 1 should thus be more than adequate to provide sufficient power to detect a significant change. Further power will be obtained through having baseline control data and from stratifying the analysis by hospital wards.
Statistical analysis – point prevalence data
Statistical analysis will be undertaken to determine differences in the prevalence of IDCs between Health District 1 (post) and Health District 2 (no intervention). A mixed methods analysis will compare pre and post data within the groups, across the time frame. Within group data will be stratified according to wards to allow for variation in the case mix of patients between wards. If differences in patient demographics are detected at baseline these will be controlled for in the between group analyses. Data linkage will be used to determine LoS and CAUTI rates for patients at each time point.
Qualitative analysis
All focus group interviews will be digitally audio-recorded and later transcribed verbatim by a professional transcriber/research assistant. Data will be analysed, coded and themed to low-level themes [44]. Cross-checking of coding will occur within the research team, and emerging themes will be shared within the whole research team as a check on credibility.
Using a mixed methods approach, the quantitative data from the point prevalence survey and the clinician survey will be analysed to inform the questions for the focus groups.
Economic evaluation
The economic evaluation will be based on a cost-effectiveness analysis to determine whether the multifaceted care intervention is more cost-effective than usual care in reducing CAUTI amongst hospital inpatients. A healthcare provider perspective will be adopted. International guidelines for conducting economic evaluations, as recommended by Drummond et al.[45], and Husereau et al. [46] will be followed. Resource use will be identified using a short data collection instrument. Cost related data collected from usual care and intervention arms will include: materials used for catheterisation, proportion of patients receiving IDC, CAUTI rates, LoS for patients diagnosed with CAUTI, and CAUTI treatment expenses (e.g. antibiotics).
The measure of effect will be based on the change in the rate of CAUTI between the usual care and intervention groups. If the expected intervention benefit is demonstrated in the trial, the measure of effect will be the cases of CAUTI avoided due to the intervention. The economic analysis will identify the cost to avoid an additional case of CAUTI. The reportable outcomes will be average cost-effectiveness and incremental cost-effectiveness ratios. A sensitivity analysis will be conducted to explore the robustness of the results to the uncertainty around parameters used in the model. The results will be interpreted in a broader decision making framework that includes acceptability and sustainability of the intervention. The economic sustainability of the intervention will be based on the cost and effect of delivering the intervention in a wider setting. The analysis will also report the resources required to implement the intervention in other localities. This information is relevant to policy makers because it reflects the resources required by other Health Districts to implement the intervention.
Discussion
A review of the literature highlighted a lack of interventional studies aiming to reduce IDC use or CAUTI rates in the Australian context. Internationally, there is a sparsity of studies using a control design in CAUTI intervention evaluations.
This study will add to the evidence-base through enhancing understanding of interventions to reduce CAUTI, using a control design to reduce secular effects.
Using the TIDieR framework, implementation strategies have been explicitly outlined, enabling easier replication of the intervention and implementation strategies. The use of a mixed methods approach will provide a platform to explore in-depth the existing barriers and enablers related to implementing practice change.
Ultimately, this study will improve patient safety through implementation and a robust evaluation of clinical practice and practice change.
Acknowledgements
Not applicable.
Funding
Funding for the project has been granted through the NSW Ministry of Health Translational Research Grants.
Availability of data and materials
Not applicable.
Authors’ contributions
VP and MG contributed to the development of research conception, design and methods and have significantly critically revised and contributed to the content in this manuscript. LG contributed to the initial drafting of this manuscript. BS contributed significantly to the development of the design and power and sample size calculation. WW contributed to the development of research conception, design and methods. TO contributed to the development of research conception, design and methods, and critically revised the content in this manuscript. AS contributed to the economic evaluation. All authors have read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Ethical approval has been granted through Hunter New England Local Health District’s and Central Coast Local Health District’s Human Research Ethics Committees (Ref 16/10/19/5.09 and 1016-097C respectively).
Data will be stored on a secure network, protected by password and only accessible to members of the research team. A formal data monitoring committee was not warranted as there are known minimal risks. The collection and management of data is overseen by the research team.
Patients will be receiving routine care in alignment with best practice principles in relation to IDC usage so consent is not required from patients. Healthcare professional participants will give informed consent to participate in focus groups and consent will be implied when they undertake the survey.
The conduct of the research is being overseen by a steering committee made up of experienced researchers and clinicians.
There will be a report to the NSW Health funding agency and the participating health districts, journal publications and conference presentations. Evaluation and outcomes will be disseminated to colleagues within and outside the participating health districts to inform sustainability of best practice related to urinary catheterisation and CAUTI prevention.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AHRQ
Agency for Healthcare Research and Quality
- CAUTI
Catheter-associated urinary tract infection
- CEC
Clinical Excellence Commission
- CUSP
Comprehensive Unit-based Safety Program
- HAI
Healthcare-associated infection
- IDC
Indwelling urinary catheter
- LoS
Length of stay
- TIDieR
Template for Intervention Description and Replication
- UTI
Urinary tract infection.
Additional file
Nurse-initiated IDC assessment and removal decision flowchart. (DOCX 152 kb)
Contributor Information
Vicki Parker, Email: vparker3@une.edu.au.
Michelle Giles, Email: Michelle.giles@hnehealth.nsw.gov.au.
Laura Graham, Email: Laura.graham@hnehealth.nsw.gov.au.
Belinda Suthers, Email: Belinda.suthers@hnehealth.nsw.gov.au.
Wendy Watts, Email: Wendy.watts@hnehealth.nsw.gov.au.
Tony O’Brien, Email: tony.obrien@newcastle.edu.au.
Andrew Searles, Email: Andrew.Searles@hmri.org.au.
References
- 1.Meddings J, Rogers M, Krein S, Fakih M, Olmsted R, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: An integrative review. BMJ Qual Saf. 2014;23:277–289. doi: 10.1136/bmjqs-2012-001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allegranzi B, Bagheri Nejad S, Garcia Castillejos G, Kilpatrick C, Kelley E, Mathai E. Report on the burden of endemic health care-associated infection worldwide: A systematic review of the literature. World Health Organization. 2011. http://apps.who.int/iris/bitstream/10665/80135/1/9789241501507_eng.pdf. Accessed 25 Oct 2016.
- 3.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray S, Thompson D, Wilson L, Fridkin S. Multistate Point-Prevalence Survey of Health Care–Associated Infections. N Engl J Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000;21(8):510–515. doi: 10.1086/501795. [DOI] [PubMed] [Google Scholar]
- 5.Association for Professionals in Infection Control and Epidemiology (APIC) Guide to preventing catheter-associated urinary tract infections. 2014. [Google Scholar]
- 6.Gokula RRM, Hickner JA, Smith MA. Inappropriate use of urinary catheters in elderly patients at a midwestern community teaching hospital. Am J Infect Control. 2004;32(4):196–199. doi: 10.1016/j.ajic.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Healthcare Infection Control Practices Advisory Committee: Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319–326. doi: 10.1086/651091. [DOI] [PubMed] [Google Scholar]
- 8.Yiou R, Audureau E, Loche CM, Dussaud M, Lingombet O, Binhas M. Comprehensive evaluation of embarrassment and pain associated with invasive urodynamics. Neurourol Urodyn. 2015;34(2):156–160. doi: 10.1002/nau.22521. [DOI] [PubMed] [Google Scholar]
- 9.Saint S, Olmsted RN, Fakih MG, Kowalski CP, Watson SR, Sales AE, Krein SL. Translating health care–associated urinary tract infection prevention research into practice via the bladder bundle. Jt Comm J Qual Patient Saf. 2009;35(9):449. doi: 10.1016/S1553-7250(09)35062-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell BG, Ferguson JK, Anderson M, Sear J, Barnett A. Length of stay and mortality associated with healthcare-associated urinary tract infections: a multi-state model. J Hosp Infect. 2016;93(1):92–99. doi: 10.1016/j.jhin.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Umscheid C, Mitchell M, Doshi J, Agarwal R, Williams K, Brennan P. Estimating the Proportion of Healthcare-Associated Infections That Are Reasonably Preventable and the Related Mortality and Costs. Infect Control Hosp Epidemiol. 2011;32(2):101–114. doi: 10.1086/657912. [DOI] [PubMed] [Google Scholar]
- 12.Jackson T, Nghiem HS, Rowell D, Jorm C, Wakefield J. Marginal costs of hospital-acquired conditions: information for priority-setting for patient safety programmes and research. J Health Serv Res Policy. 2011;16(3):141–146. doi: 10.1258/jhsrp.2010.010050. [DOI] [PubMed] [Google Scholar]
- 13.Meddings JA, Reichert H, Rogers MAM, Saint S, Stephansky J, McMahon LF. Impact of Non-Payment for Hospital-Acquired Catheter-Associated Urinary Tract Infection: A Statewide Analysis. Ann Intern Med. 2012;157(5):305–312. doi: 10.7326/0003-4819-157-5-201209040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sampathkumar P, Barth JW, Johnson M, Marosek N, Johnson M, Worden W, Lembke J, Twing H, Buechler T, Dhanorker S, Keigley D, Thompson R. Mayo Clinic Reduces Catheter-Associated Urinary Tract Infections Through a Bundled 6-C Approach. Jt Comm J Qual Patient Saf. 2016;42(6):254–261. doi: 10.1016/S1553-7250(16)42033-7. [DOI] [PubMed] [Google Scholar]
- 15.Andreessen L, Wilde MH, Herendeen P. Preventing catheter-associated urinary tract infections in acute care: the bundle approach. J Nurs Care Qual. 2012;27(3):209–217. doi: 10.1097/NCQ.0b013e318248b0b1. [DOI] [PubMed] [Google Scholar]
- 16.Carter NM, Reitneier L, Goodloe LR. An Evidence-Based Approach To the Prevention of Catheter-Associated Urinary Tract Infections. Urol Nurs. 2014;34(5):238–245. [PubMed] [Google Scholar]
- 17.Clarke K, Tong D, Pan Y, Easley KA, Norrick B, Ko C, Wang A, Razavi B, Stein J. Reduction in catheter-associated urinary tract infections by bundling interventions. Int J Qual Health Care. 2013;25(1):43–49. doi: 10.1093/intqhc/mzs077. [DOI] [PubMed] [Google Scholar]
- 18.Harris TA. Changing practice to reduce the use of urinary catheters. Nursing. 2010;40(2):18–20. doi: 10.1097/01.NURSE.0000367857.98069.ad. [DOI] [PubMed] [Google Scholar]
- 19.Saint S, Greene MT, Krein SL, Rogers MA, Ratz D, Fowler KE, Edson BS, Watson SR, Meyer-Lucas B, Masuga M, et al. A Program to Prevent Catheter-Associated Urinary Tract Infection in Acute Care. N Engl J Med. 2016;374(22):2111–2119. doi: 10.1056/NEJMoa1504906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Titsworth WL, Hester J, Correia T, Reed R, Williams M, Guin P, Layon AJ, Archibald LK, Mocco J. Reduction of catheter-associated urinary tract infections among patients in a neurological intensive care unit: a single institution’s success. J Neurosurg. 2012;116(4):911–920. doi: 10.3171/2011.11.JNS11974. [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Balmer F, Friedli-Wuthrich H, Muhlemann K. Reduction of urinary catheter use and prescription of antibiotics for asymptomatic bacteriuria in hospitalised patients in internal medicine: before-and-after intervention study. Swiss Med Wkly. 2013;143:w13796. doi: 10.4414/smw.2013.13796. [DOI] [PubMed] [Google Scholar]
- 22.Fakih MG, Watson SR, Greene MT, Kennedy EH, Olmsted RN, Krein SL, Saint S. Reducing inappropriate urinary catheter use: a statewide effort. Arch Intern Med. 2012;172(3):255–260. doi: 10.1001/archinternmed.2011.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fakih MG, Rey JE, Pena ME, Szpunar S, Saravolatz LD. Sustained reductions in urinary catheter use over 5 years: Bedside nurses view themselves responsible for evaluation of catheter necessity. Am J Infect Control. 2013;41(3):236–239. doi: 10.1016/j.ajic.2012.04.328. [DOI] [PubMed] [Google Scholar]
- 24.Gray M, Skinner C, Kaler W. External Collection Devices as an Alternative to the Indwelling Urinary Catheter: Evidence-Based Review and Expert Clinical Panel Deliberations. J Wound Ostomy Continence Nurs. 2016;43(3):301–307. doi: 10.1097/WON.0000000000000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray D, Nussle R, Cruz A, Kane G, Toomey M, Bay C, Ostovar GA. Effects of a catheter-associated urinary tract infection prevention campaign on infection rate, catheter utilization, and health care workers’ perspective at a community safety net hospital. Am J Infect Control. 2016;44(1):115–116. doi: 10.1016/j.ajic.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Miller B, Krein S, Fowler K, Belanger K, Zawol D, Lyons A, Bye C, Rickelmann M, Smith J, Chenoweth C, Saint S. A Multimodal Intervention to Reduce Urinary Catheter Use and Associated Infection at a Veterans Affairs Medical Center. Infect Control Hosp Epidemiol. 2013;34(6):631–633. doi: 10.1086/670624. [DOI] [PubMed] [Google Scholar]
- 27.Parry MF, Grant B, Sestovic M. Successful reduction in catheter-associated urinary tract infections: focus on nurse-directed catheter removal. Am J Infect Control. 2013;41(12):1178–1181. doi: 10.1016/j.ajic.2013.03.296. [DOI] [PubMed] [Google Scholar]
- 28.Flodgren G, Conterno LO, Mayhew A, Omar O, Pereira CR, Shepperd S: Interventions to improve professional adherence to guidelines for prevention of device-related infections. Cochrane Database of Systematic Reviews. 2013(3). Art. No.: CD006559. doi: 10.1002/14651858.CD006559.pub2. [DOI] [PubMed]
- 29.Wynne R, Patel M, Pascual N, Mendoza M, Ho P, Qian D, Thangavel D, Law L, Richards M, Hobbs L. A single centre point prevalence survey to determine prevalence of indwelling urinary catheter use and nurse-sensitive indicators for the prevention of infection. Healthcare Infection. 2014;19(1):13–9.
- 30.So K, Habashy D, Doyle B, Chan L. Indwelling urinary catheters: pattern of use in a public tertiary-level Australian hospital. Urol Nurs. 2014;34(2):69–73. [PubMed] [Google Scholar]
- 31.Harley G, Yeo AL, Stuart RL, Dendle C. A real-life snapshot of the use and abuse of urinary catheters on general medical wards. Infect Control Hosp Epidemiol. 2011;32(12):1216–1218. doi: 10.1086/662625. [DOI] [PubMed] [Google Scholar]
- 32.Giles M, Watts W, O’Brien A, Berenger S, Paul M, McNeil K, Bantawa K. Does our bundle stack up! Innovative nurse-led changes for preventing catheter-associated urinary tract infection (CAUTI) Healthcare Infection. 2015;20(2):62–71. doi: 10.1071/HI14035. [DOI] [Google Scholar]
- 33.Gardner A, Mitchell B, Beckingham W, Fasugba O. A point prevalence cross-sectional study of healthcare-associated urinary tract infections in six Australian hospitals. BMJ Open. 2014;4(7). [DOI] [PMC free article] [PubMed]
- 34.Rohrmann S, Overvad K, Bueno-de-Mesquita HB, Jakobsen MU, Egeberg R, Tjønneland A, et al. Meat consumption and mortality - results from the European Prospective Investigation into Cancer and Nutrition. BMC Med. 2013;11:63. [DOI] [PMC free article] [PubMed]
- 35.NSW Health. Adult Urethral Catheterisation for Acute Care Settings. http://www1.health.nsw.gov.au/PDS/pages/doc.aspx?dn=GL2015_016. Accessed 25 Oct 2016.
- 36.Clinical Excellence Commission (CEC). Catheteter-associated urinary tract infections (CAUTIs). http://www.cec.health.nsw.gov.au/patient-safety-programs/adult-patient-safety/cauti-prevention. Accessed 25 Oct 2016.
- 37.Health Education & Training Insitute (HETI). Inserting an Indwelling Urinary Catheter. http://www.heti.nsw.gov.au/Courses/Inserting-an-Indwelling-Urinary-Catheter/. Accessed 25 Oct 2016.
- 38.StatCorp . Stata Statistical Software: Release 14. College Station: StataCorp LP; 2015. [Google Scholar]
- 39.Institute for Healthcare Improvement. Bundle up for safety. http://www.ihi.org/resources/Pages/ImprovementStories/BundleUpforSafety.aspx. Accessed 25 Oct 2016.
- 40.Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348. [DOI] [PubMed]
- 41.Steinmo S, Fuller C, Stone SP, Michie S. Characterising an implementation intervention in terms of behaviour change techniques and theory: the ‘Sepsis Six’ clinical care bundle. Implement Sci. 2015;10(1):111. doi: 10.1186/s13012-015-0300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fakih M, Krein S, Edson B, Watson S, Battles J, Saint S. Engaging healthcare workers to prevent catheter-associated urinary tract infection and avert patient harm. Am J Infect Control. 2014;42:S223–S229. doi: 10.1016/j.ajic.2014.03.355. [DOI] [PubMed] [Google Scholar]
- 43.Damschroder LJ, Banaszak-Holl J, Kowalski CP, Forman J, Saint S, Krein SL. The role of the “champion” in infection prevention: results from a multisite qualitative study. Qual Saf Health Care. 2009;18(6):434–440. doi: 10.1136/qshc.2009.034199. [DOI] [PubMed] [Google Scholar]
- 44.Bazeley P. Qualitative Data Analysis: Practical Strategies. Australia: Research Support Pty Ltd; 2013. [Google Scholar]
- 45.Drummond MF, Sculpher GW, Torrance GW, O’Brien B, Stoddart GL. Methods for the Economic Evaluation of Health Care Programs. Oxford: Oxford University Press; 2005. [Google Scholar]
- 46.Husereau D, Drummond MF, Petrou S, Carswell C, Moher D, Greenberg F, Augustovski A, Briggs J, Mauskopf J, Loder E. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) - Explanation and Elaboration: A report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–250. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Tenke P, Koves B, Johansen T. An update on prevention and treatment of catheter-associated urinary tract infections. Curr Opin Infect Dis. 2014;27(1):102–107. doi: 10.1097/QCO.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 48.Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, Saint S, Schaeffer AJ, Tambayh PA, Tenke P, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 49.Oman KS, Makic MB, Fink R, Schraeder N, Hulett T, Keech T, Wald H. Nurse-directed interventions to reduce catheter-associated urinary tract infections. Am J Infect Control. 2012;40(6):548–553. doi: 10.1016/j.ajic.2011.07.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


