Abstract
Background:
Unfortunately, despite an increase in medical knowledge, survival rates of head and neck cancers (HNCs) have not been observed to improve greatly. This is true, especially in tumors located in obscure primary sites or late presentation.
Aim:
The purpose of this study is to assess the epidemiologic pattern of HNCs and to evaluate its emerging trends and patterns in Lagos state.
Subjects and Methods:
A retrospective study was conducted from 2003 to 2013 that analyzed histologically diagnosed cases of HNC at the Pathology Departments of the two tertiary teaching hospitals in Lagos. Data analysis was performed using the Statistical Package for the Social Sciences (version 20) statistical software.
Results:
One thousand and eighty-three cases of head and neck malignancies were recorded. A female: male ratio of 1.01:1 was reported with mean age of 39.6 (standard deviation 21.1) years. The oral cavity was the most affected anatomic site (21.2%, 230/1083) in the period under review. Malignant epithelial tumors accounted for 72% (779/1083) of cases seen. Carcinomas were the most common histological variant seen (67%, 726/1083) and squamous cell carcinoma accounted for 58% (421/726) of carcinomas recorded. Oral cavity malignancies (21.8%, 118/540) were the most common in males while thyroid malignancies (28.5%, 155/543) were the most seen in females. In children (≤15 years), the most common histologic findings were carcinomas (42.3%; 77/182) and retinoblastomas (23.6%; 43/182).
Conclusion:
Epithelial malignancies were the most common malignancy in the study, and the oral cavity appears to be the increasingly predominant site for HNCs. A changing pattern in gender predominance, age distribution, and frequency with histological variants and anatomical sites was also observed in this study.
Keywords: Epidemiology, Head and neck cancer, Prevalence
Introduction
Cancer is rapidly becoming a public health crisis in low- and middle-income countries.[1] It remains a leading noncommunicable disease in Africa, and it is also emerging as a great burden when compared to infections that are ravaging the continent. The triad of ignorance, poverty, and poor health-seeking behavior makes Africa vulnerable to the cancer burden, irrespective of gender and age. Approaches to minimize the burden of cancer in Sub-Saharan Africa in the past few years have had little success. Reasons include low awareness of the cancer burden and a poor understanding of the potential for preventing cancer.[2]
Head and neck cancers (HNCs) are a wide range of malignant tumors found in anatomical sites, such as the oral cavity, ear, scalp, nasal cavities, paranasal sinuses, nasopharynx, hypopharynx, oropharynx, salivary glands, facial soft tissue, malignant neck masses, thyroid, and eye.[3] They present with various biological patterns and are unique due to delicate structures in the head and neck region.[3,4]
The occurrence of HNC malignant neoplasms is largely due to multiple factors which can be broadly classified as environmental and genetic.[5] Environmental factors include usage and consumption of tobacco and alcohol in various forms, improper nutrition, as well as oncogenic virus infection, such as human papilloma virus in laryngeal cancer.[3,6] Other causes include excessive consumption of Chinese-style salted foods, industrial pollution, medication use, and race.[7,8] Interactions between these factors have been reported to account for the development of HNC.[3,6,7,8]
The lesions clinically present in different ways depending on the anatomical site of tumor origin and stage of the disease. Early-stage HNCs often present with diverse and on occasion unspecific signs and symptoms. However, a number of HNCs present late due to the obscure nature of their anatomical site of occurrence, thereby making early diagnosis and management difficult.[4]
Various histological types of HNC have been identified;[3,5,6,9] with squamous cell carcinoma (SCC) reported to account for over 90% of HNCs[5,6,9,10] and lymphomas for 10% constituting the second most common primary malignancy of the head and neck region.[3]
Surgery as well as adjuvant radiotherapy and chemotherapy remains the mainstay of treatment for early operable HNCs. Unfortunately, despite an increase in medical knowledge, survival rates, especially with tumors located in obscure primary sites or late stage tumors, have not been observed to improve greatly.[4]
Data from scientific literature demonstrate sparse data on HNCs in Africa.[4] Reports from Africa are also limited to a few hospital cancer registries. It is, therefore, difficult to extrapolate the true pattern in these countries.[11] A 2-year global epidemiological survey observed a total of <1.0% data on HNCs from African studies.[12]
As far as we know, the precise prevalence of HNC in Nigeria remains unknown. The population and land size of Nigeria is large, and the customs and practices are just as varied. The climatic conditions from one place to the other also vary considerably. The reports from various centers across the country as reviewed by da Lilly-Tariah et al.[13] have been described as “hardly a true reflection of the cancer pattern of any particular tribe or groups.” The nature of the studies such as the study design (mostly retrospective in nature), small sample size, and short duration of study are some of the factors that make it difficult to make out any clear-cut disease pattern.
HNCs are reported to constitute 5%–50% of all cancers globally, and 5%–8% of total body cancers in Europe and America. In India, HNC constitutes about 30% of all cancers.[13] In Nigeria, most reports detailed nasopharyngeal cancers as the most common HNCs. Nevertheless, studies from Ilorin and Ile-Ife reported nasal/paranasal cancers and oral cancers as the most frequently occurring HNC, respectively.[13,14,15,16,17,18] In addition, patterns varied at different times in the same center, as seen in Jos with Bhatia, Otoh et al., and Adoga et al.,[7,14,19] as well as Ibadan.[3,13,20] Perhaps, this attests to the possibility of a change in the pattern of HNCs over time, as well as geographical variations in pattern.
The purpose of this study is to assess the epidemiologic pattern of HNCs in Lagos state. It is also to evaluate the trends and patterns with respect to age, anatomic sites, and histopathologic distribution in the state. Data obtained will serve as a valuable tool used to promote the awareness of HNC; it will also set the groundwork for a population-based study to determine the regional incidence of HNC in the state and country as a whole.
Subjects and Methods
This study was carried out at Lagos University Teaching Hospital and Lagos State University Teaching Hospital retrospectively from 2003 to 2013. Data were retrieved from histopathological records of specimens sent to the Departments of Oral and Maxillofacial Pathology/Biology and the Anatomic and Molecular Pathologies of the two institutions. These two tertiary health institutions receive histopathologic specimens from public and private health-care centers within Lagos state and other health institutions from neighboring states. Ethical approval for the study was obtained from the Health Research and Ethical Committee of both institutions.
All cases of primary malignant neoplasms of the oral cavity, ear, scalp, nasal cavities, paranasal sinuses, nasopharynx, hypopharynx, oropharynx, salivary glands, facial soft tissue, malignant neck masses, thyroid, and eye were retrieved. Information on age and gender of the patients, the anatomic site, histologic diagnosis, classification, and tissue of origin of the cancers were collected. However, cases of secondary metastases, intracranial region, parathyroid gland, trachea, esophagus, benign neoplasms of the head and neck region, as well as patients with incomplete demographic data or whose histological diagnosis could not be verified were excluded from the study.
Data retrieved were grouped and classified based on the gender of patients, tumor diagnosis, anatomical site, and tumor histology. Anatomical site groupings for the cases were done using the standardized classification for HNC International Classification of Diseases 10. HNCs were grouped into malignant neoplasms of epithelial, connective, and neural tissue origin showing histological features of malignancy (M – 3000/3). Patients seen were classified based on age into children (≤15 years and adults >15 years).
Data analysis was performed using the Statistical Package for the Social Sciences version 20.0 (IBM, USA). Simple descriptive statistics and Chi-square tests were used as necessary to present the data. Statistical significance was inferred at P ≤ 0.05.
Results
In the period under study (2003–2013), a total number of 1083 cases of head and neck malignancies were recorded. Incidence was estimated at 99 cases/year within the state. The majority of these tumors were malignant epithelial tumors accounting for 72% (781/1083), while mesenchymal malignant tumors made up 28% (302/1083) of the cases seen. A marginal female predominance (50.1%, 543/1083) over males (49.9%, 540/1083) was seen, with a ratio of 1.01:1.
Age
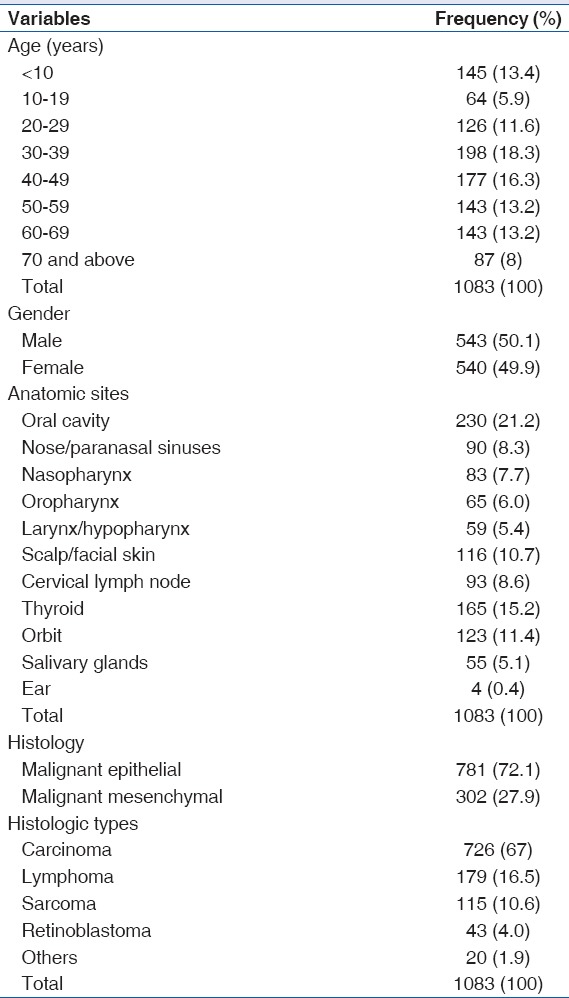
The age of cases seen ranged from 3 months to 97 years. The majority of cases were adults (83.2%, 901/1083), while 16.8% (182/1083) were children below the age of 15 years. The lowest and peak incidence of tumors were seen in the second (5.9%, 64/1083) and fourth (18.3%, 198/1083) decades, respectively [Table 1]. The peak incidence of tumors was observed for both malignant epithelial and mesenchymal tumors in the same decade (30–39 years). After the peak incidences in the fourth decade, there was a steady decline in the incidence with the incidence for patients ≥70 years at 8% (87/1083).
Table 1.
Demographic data, anatomical sites, and histological variants
Anatomical site distribution
The most common anatomical site for HNC in this study was the oral cavity accounting for 21.2% (230/1083), this was followed by the thyroid (15.2%, 165/1083) and orbit (11.4%, 123/1083). Nasopharyngeal cancer accounted for 7.7% (83/1083) of all cases seen, while nose/paranasal sinuses accounted for 8.3% (90/1083). Salivary gland malignancies were rare, with 5.1% (55/1083) prevalence rate in this study [Table 2]. The oral cavity was also the most common anatomical site in patients above 50 years. Thyroid malignancies were the most seen in females (28.5%, 155/543), while the oral cavity was the most affected anatomical site in males (21.8%, 118/540). The thyroid was the least affected site in males (1.9%, 20/540) and the larynx was the least affected in females (0.9%, 5/543) [Tables 3 and 4].
Table 2.
Relationship between anatomic sites and age of patients
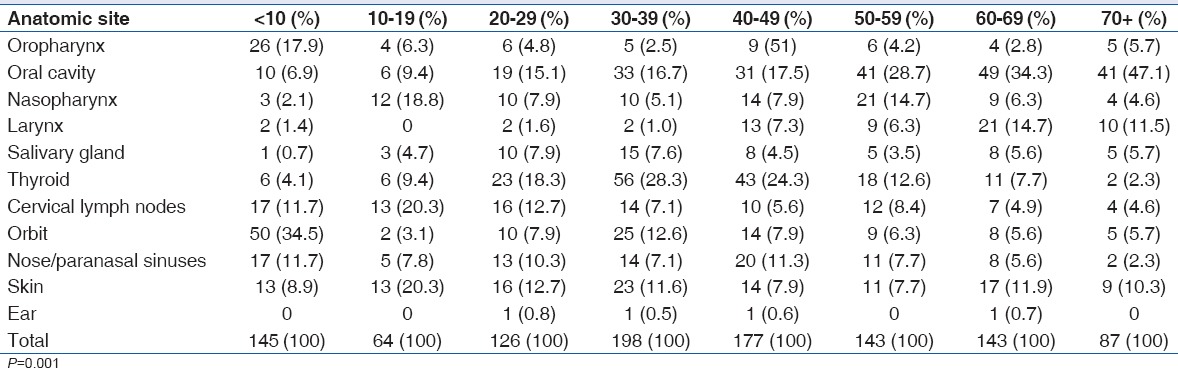
Table 3.
Relationship between gender and anatomical sites
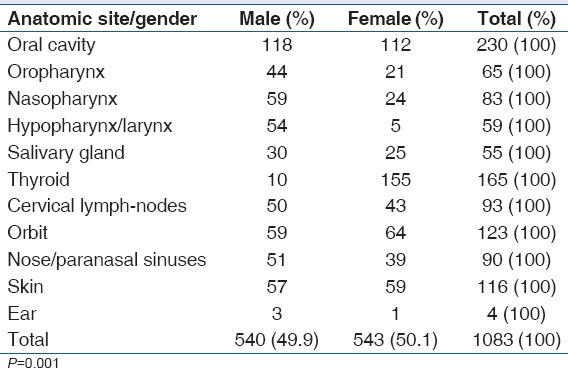
Table 4.
Relationship between anatomical sites and histologic variants
Histological distribution
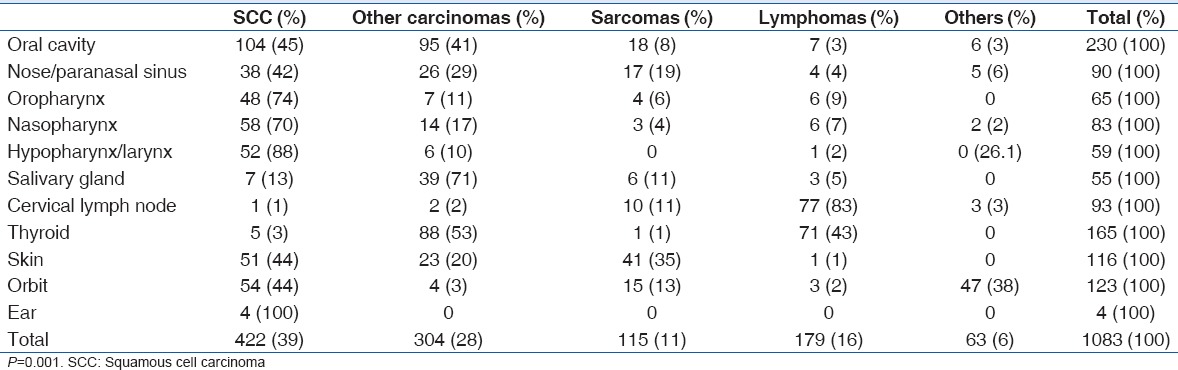
In general, carcinomas accounted for the highest number of recorded cases (67%, 726/1083), followed by lymphomas (16.5%, 179/1083) and sarcomas (10.6%, 115/1083) [Table 3]. SCC accounted for 58% (421/726) of carcinomas recorded, while adenocarcinomas reported were 13.2% (96/726) of all carcinomas. Follicular lymphomas (48%, 86/179) were the most common histologic finding among lymphomas, with non-Hodgkin's (26.8%, 48/179) and Hodgkin's lymphomas (16.7%, 30/179). Among the sarcomas diagnosed, fibrosarcomas and liposarcomas both detailed 20.9% (9/43) each, while rhabdomyosarcoma cases were 14% (6/43). Retinoblastoma accounted for 4% (43/1083) of histologic variations seen and was found exclusively in the orbit in cases ≤15 years.
In children, the most common histologic findings were carcinomas (41.8%, 76/182), retinoblastomas (23.6%, 43/182), sarcomas (18%, 33/182), and lymphomas (14.8%, 27/182). In adults, carcinomas accounted for 72.1% (650/901) of malignancies, while lymphomas accounted for 16.8% (152/901) and sarcomas 9.1% (82/901).
The female gender had a higher rate of sarcomas (51.3%, 59/115) and lymphomas (62%, 111/179) compared to the male (P = 0.001). SCC accounted for 30.9% (168/543) of cases seen in the female gender, followed by follicular lymphomas (13.6%, 74/543). In the male gender, SCC was reported in 46.9% (253/540) of the cases seen and sarcomas in 10.4% (56/540) of cases.
Discussion
Sub-Saharan Africa has a disproportionate burden of disease and the overall disease burden attributable to cancer is rising.[2] The devastating psychosocial effects on the individual, family, and society are enormous. Understanding the pattern of HNC statewide will have important implications for prevention and could form cancer management policies.
In this study, we evaluated statewide patterns in the prevalence and histologic trends for HNC using histopathologic data from the two tertiary hospitals in Lagos state. We compared and contrasted trends for HNC reported in a previous study in the state over a decade ago,[17] with those found in our study. This was to investigate any changes in histological patterns, age, and gender affectation, as well as anatomic sites within a hospital-based population in the state. The current study accounts for the highest prevalence and yearly incidences recorded so far within the country. This may be attributed to the inclusion of certain anatomical sites which could have been excluded from other studies, such as thyroid and eye. A diversity of cancers in anatomical sites included under the broad group “HNC” by various authors has contributed to disparities in recording and comparison of the geographical distribution of HNCs.
Perusal of Nigerian scientific literature has also shown variations and inconsistencies in the anatomical site groupings of HNC.[3,10] For example, a previous study conducted in Jos classified HNC using upper aerodigestive tract only,[21] whereas in Ife, the ocular cavity, facial soft tissue malignancy, and neck masses were used for classification.[16] Nwawolo et al.[17] and several other authors[20,22] included neither cervical esophageal, thyroid nor eye malignancies in this group of lesions. However, these sites (except the cervical esophageal) were included in our study. Furthermore, Adeyemi et al.[3] did not separate oral cancers from oropharyngeal cancers in their report. Such variations and inconsistencies may result in erroneous compilation of data. It is, therefore, necessary to standardize the method of anatomical site groupings of HNC. Furthermore, previous studies reported in the country have been single institutional studies,[13] and this may also account for the low prevalence as compared to our result. A multiinstitutional study was necessary within this region, as the state being investigated in the study has two tertiary health institutions with facilities and staff for the diagnosis and management of HNC.
This study reveals that HNCs are more common in adults than children. This is consistent with most studies within the country.[13] In children and adults, SCC was the most common histological variant. This contrasts with other studies in Jos and Ibadan where lymphomas accounted for the most frequent variant in children.[7,20] However, our results are in consonant with a previous study in Lagos.[17] The orbit and oral cavity were the most common anatomical sites in children, while the oral cavity was the most common in adults. HNCs occurred less frequently in the second decade. There was a rise in occurrence which peaked in the fourth decade and began a steady decline. This finding was similar to Nwawolo et al.'s[17] finding within the state. However, this differed slightly from the other reports from Nigeria, particularly the Southwestern region where Lagos is situated.[20] The mean age of 39.4 years obtained is similar to studies from Gombe and Ibadan where a mean age of 38.3 and 43.9 was detailed, respectively.[3,22] Significant increases in HNC was also observed among younger age groups; this corresponds with reports in most countries detailing an increasing incidence being statistically significantly stronger at ages younger than 60 years.[23] This may be attributed to increasing industrialization within the state, leading to exposure of the working adult population to environmental risk factors.
The gender ratio in our study indicated a female majority. This contrasts with most other studies in Nigerians which detailed a male predominance in HNC cases. da Lilly-Tariah et al[13] in a meta-analysis review of 27 relevant articles from 1968-2008 reported a male: female ratio that ranged from 1:1 to 2.3:1. This is also supported by several foreign reports.[10,20] Nevertheless, a study in Ilorin detailed a female predominance in consonance with our report.[15] The authors believe that this finding may be attributed to the better health-seeking behavior of females, and presume the esthetic challenges posed by HNCs will motivate females to seek treatment more often. This changing trend will however be further clarified by more studies on the subject.
The oral cavity was the most affected anatomical site in males from the present study. The authors are of the opinion that this may be due to the higher rate of exposure of the male folk to risk factors such as alcohol and smoking. In the Nigerian population, smoking and the use of tobacco products is more prevalent among males as compared to females.[3,24] The least common site in females was the hypopharynx/larynx. This could be attributed to the fact that habits such as smoking and use of alcoholic beverages that have been associated with the occurrence of cancer at the hypopharynx/larynx are more prevalent among the male gender in our society.[3]
Malignant epithelial tumors accounted for a majority of all tumors across all age groups and gender. Which is comparable to an earlier study in Lagos by Nwawolo et al.[17] This predominance of malignant epithelial tumors in the head and neck in this study is in line with other reports.[13] Nwawolo et al.[17] reported sarcomas as the second most common type, in contrast to our study where lymphomas accounted for the second most frequent type followed by sarcomas. Nevertheless, our findings agree with numerous studies in other regions of the country[13,14,20,22,24] which followed this order. In addition, sarcomas were found to have a female predominance in contrast to a previous local study which reported a male predominance.[20] An increase in thyroid malignancies was also observed in this study, from 7.3% by Nwawolo et al. to 15.2% in this study; sarcomas followed the same trend from 6.6% in the previous study to 10.6% in this report.[17] The authors believe that this rise can be attributed to the broad picture obtained due to the multicenter data obtained in this study.
In the present study, the oral cavity was the most affected anatomic site in the period under review. Other studies conducted in the Southwestern geographical region of Nigeria have reported similar findings,[3,16] except studies whose period of review extended well into the late 1980s and early 1990s.[17,20] A change in lifestyle of the population and increased urbanization may be responsible for this phenomenon.[13] As Nigeria, particularly the Southwestern geographical region, continues to industrialize, the role of carcinogenic agents arising from increased industrialization cannot be ignored. The thyroid was the second most common prevalence site for HNCs from our findings with follicular carcinoma being the most common variant. This has been identified as a common finding for HNC in several local studies from different geographical zones such as North Central (Jos) and Northeast (Gombe).[7,22] Thyroid malignancies were also found to occur most commonly in the fourth decade, contrasting with prior reports from Ife, which detailed a peak in the third and fifth decades; these reports however indicated a female majority in consonance with our study.[16] We believe the thyroid sites may be underreported in HNCs due to its repeated exclusion from several HNC epidemiologic studies conducted locally.[17,20,22,24]
The nose/paranasal sinuses have been reported to be a common site for HNCs.[15,19,20,24] Although, in our study, it accounted for only 8.3% of cases seen, this was a decrease from 13.6% reported in Lagos over a decade ago.[17] This decrease may be attributed to a variation in the grouping of HNCs, where several anatomic sites may have been excluded, making the sites available (if fewer) more in percentage frequency. Furthermore, the prior study in Lagos reviewed the radiotherapy records.
Nasopharyngeal cancer accounted for 7.7% of all HNCs in this study. This was contradicted by the previous study in Lagos, in which nasopharyngeal cancers accounted for the most common anatomical site (16.8%).[17] Nasopharyngeal cancers were found to be the second most common among the age range 50–59 years (14.7%) and more common in the male gender from our study; this is supported by existing local data.[13,14,19,20,22,24] Nevertheless, there appears to be a change in the age of cases toward an older age group. Our studies contradict other local, regional studies which record a high incidence in the fourth and fifth decades.[3,16] Furthermore, SCC was the most common histological variant in this region.
Salivary gland cancers with a prevalence of 5.1% of all HNCs in this study were found to be relatively low compared to other studies in the Southwestern region.[15,16,20] This result however did not differ markedly from Nwawolo et al.'s report of 7.6%.[17] Mucoepidermoid carcinoma was the most common, 29% histologic variant in the salivary glands. A male predominance was reported in our study supporting findings from prior regional studies.[3,7,16] However, a change in the age of presentation was observed, as a high incidence was recorded in the third-fourth decade in our study, contrary to other studies which report a high incidence in cases above the fifth decade.[3,7,16]
Conclusion
This epidemiological summary of head and neck malignancies in Lagos state, Southwestern Nigeria, though presenting with certain changing trends, is not entirely different from patterns in other parts of the country and the world. Epithelial malignancies are more common than other lineages in the head and neck. The oral cavity still appears to be the increasingly predominant site for HNCs as it is in most other studies. A changing pattern in gender predominance, age distribution, and frequency with various histological variants and anatomical sites was however observed in this study. It will be beneficial to conduct this sort of study intermittently to monitor the changing trends of HNC so that apt attention can be accorded to the anatomically and histologically predominant types.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors gratefully acknowledge the support and contribution of the Oral and General Pathology Departments of Lagos State University Teaching Hospital and Lagos University Teaching Hospital. We acknowledge Mr. Reuben Ogunleke for his support in data collection at the Lagos State University Teaching Hospital and Dr. Enabulele for reading the first draft of the paper.
References
- 1.Kingham TP, Alatise OI, Vanderpuye V, Casper C, Abantanga FA, Kamara TB, et al. Treatment of cancer in Sub-Saharan Africa. Lancet Oncol. 2013;14:e158–67. doi: 10.1016/S1470-2045(12)70472-2. [DOI] [PubMed] [Google Scholar]
- 2.Morhason-Bello IO, Odedina F, Rebbeck TR, Harford J, Dangou JM, Denny L, et al. Challenges and opportunities in cancer control in Africa: A perspective from the African Organisation for Research and Training in Cancer. Lancet Oncol. 2013;14:e142–51. doi: 10.1016/S1470-2045(12)70482-5. [DOI] [PubMed] [Google Scholar]
- 3.Adeyemi BF, Adekunle LV, Kolude BM, Akang EE, Lawoyin JO. Head and neck cancer – A clinicopathological study in a tertiary care center. J Natl Med Assoc. 2008;100:690–7. doi: 10.1016/s0027-9684(15)31343-2. [DOI] [PubMed] [Google Scholar]
- 4.Gathere S, Mutuma G, Korir A, Musibi A. Head and neck cancers four year trend at the Nairobi cancer registry. Afr J Health Sci. 2011;19:30–5. [Google Scholar]
- 5.Ruback MJ, Galbiatti AL, Arantes LM, Marucci GH, Russo A, Ruiz-Cintra MT, et al. Clinical and epidemiological characteristics of patients in the head and neck surgery department of a university hospital. Sao Paulo Med J. 2012;130:307–13. doi: 10.1590/S1516-31802012000500007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharjee A, Chakraborty A, Purkaystha P. Prevalence of head and neck cancers in the Northeast: An institutional study. Indian J Otolaryngol Head Neck Surg. 2006;58:15–9. doi: 10.1007/BF02907731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otoh EC, Johnson NW, Mandong BM, Danfillo IS. Primary head and neck cancers in Jos, Nigeria: A re-visit. West Afr J Med. 2006;25:92–100. doi: 10.4314/wajm.v25i2.28256. [DOI] [PubMed] [Google Scholar]
- 8.Mafi N, Kadivar M, Hosseini N, Ahmadi S, Zare-Mirzaie A. Head and neck squamous cell carcinoma in Iranian patients and risk factors in young adults: A fifteen-year study. Asian Pac J Cancer Prev. 2012;13:3373–8. doi: 10.7314/apjcp.2012.13.7.3373. [DOI] [PubMed] [Google Scholar]
- 9.Licitra L, Felip E. ESMO Guidelines Working Group. Squamous cell carcinoma of the head and neck: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl 4):121–2. doi: 10.1093/annonc/mdp149. [DOI] [PubMed] [Google Scholar]
- 10.Blomberg M, Nielsen A, Munk C, Kjaer SK. Trends in head and neck cancer incidence in Denmark, 1978-2007: Focus on human papillomavirus associated sites. Int J Cancer. 2011;129:733–41. doi: 10.1002/ijc.25699. [DOI] [PubMed] [Google Scholar]
- 11.Ndui MK. Mini-Thesis (PhD) South-Africa: University of Western Cape; 2011. Epidemiology of Oral Cancer in South Africa: 1996-2002. [Google Scholar]
- 12.APOCPC/UICC-ARO Cancer Registration Consortium. Cancer registration literature update (2006-2008) Asian Pac J Cancer Prev. 2008;9:165–82. [PubMed] [Google Scholar]
- 13.da Lilly-Tariah OB, Somefun AO, Adeyemo WL. Current evidence on the burden of head and neck cancers in Nigeria. Head Neck Oncol. 2009;1:14. doi: 10.1186/1758-3284-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adoga AS, John EN, Yiltok SJ, Echejoh GO, Nwaorgu OG. The pattern of head and neck malignant tumours in Jos. Highland Med Res J. 2009;8:37–41. [Google Scholar]
- 15.Ologe FE, Adeniji KA, Segun-Busari S. Clinicopathological study of head and neck cancers in Ilorin, Nigeria. Trop Doct. 2005;35:2–4. doi: 10.1258/0049475053001949. [DOI] [PubMed] [Google Scholar]
- 16.Amusa YB, Olabanji JK, Akinpelu VO, Olateju SO, Agbakwuru EA, Ndukwe N, et al. Pattern of head and neck malignant tumours in a Nigerian teaching hospital – A ten year review. West Afr J Med. 2004;23:280–5. doi: 10.4314/wajm.v23i4.28141. [DOI] [PubMed] [Google Scholar]
- 17.Nwawolo CC, Ajekigbe AT, Oyeneyin JO, Nwankwo KC, Okeowo PA. Pattern of head and neck cancers among Nigerians in Lagos. West Afr J Med. 2001;20:111–6. [PubMed] [Google Scholar]
- 18.Iseh KR, Malami SA. Pattern of head and neck cancers in Sokoto, Nigeria. Niger J Otolaryngol. 2006;3:77–83. [Google Scholar]
- 19.Bhatia PL. Head and neck cancer in Plateau state of Nigeria. West Afr J Med. 1990;9:304–10. [PubMed] [Google Scholar]
- 20.Adisa AO, Adeyemi BF, Oluwasola AO, Kolude B, Akang EE, Lawoyin JO. Clinico-pathological profile of head and neck malignancies at University College Hospital, Ibadan, Nigeria. Head Face Med. 2011;7:9. doi: 10.1186/1746-160X-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandong BM, Ngbea JA, Adoga AS. Head and neck squamous cell carcinoma: Prevention strategy. Jos J Med. 2011;5:12–6. [Google Scholar]
- 22.Akinmoladun V, Pindiga U, Akintububo O, Kokong D, Akinyamoju C. Head and neck malignant tumours in Gombe, Northeast Nigeria. J West Afr Coll Surg. 2013;3:1–15. [PMC free article] [PubMed] [Google Scholar]
- 23.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31:4550–9. doi: 10.1200/JCO.2013.50.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onotai LO, Nwogbo AC. Primary head and neck malignant tumours in Port Harcourt, Nigeria: A revisit. Int J Med Med Sci. 2012;3:122–5. [Google Scholar]


