Abstract
The human skin not only provides passive protection as a physical barrier against external injury, but also mediates active surveillance via epidermal cell surface receptors that recognize and respond to potential invaders. Primary keratinocytes and immortalized cell lines, the commonly used sources to investigate immune responses of cutaneous epithelium are often difficult to obtain and/or potentially exhibit changes in cellular genetic make‐up. Here we investigated the possibility of using salivary epithelial cells (SEC) to evaluate the host response to cutaneous microbes. Elevated secretion of IFN‐γ and IL‐12 was observed in the SEC stimulated with Staphylococcus aureus, a transient pathogen of the skin, as mono species biofilm as compared to SEC stimulated with a commensal microbe, the Staphylococcus epidermidis. Co‐culture of the SEC with both microbes as dual species biofilm elicited maximum cytokine response. Stimulation with S. aureus alone but not with S. epidermidis alone induced maximum toll‐like receptor‐2 (TLR‐2) expression in the SEC. Exposure to dual species biofilm induced a sustained upregulation of TLR‐2 in the SEC for up to an hour. The data support novel application of the SEC as efficient biospecimen that may be used to investigate personalized response to cutaneous microflora.
Keywords: biofilm, cytokines, salivary epithelial cells, TLR‐2
Introduction
Human skin is an intricate habitat for many commensal bacteria that play a significant role in protecting the host against pathogenic bacteria.1 Chronic wounds represent a disease state in which the break in the continuity of the cutaneous barrier and the disruption of the commensal microbial balance promote the development of organized microbial community or biofilm within the wound environment.2 The colonizing biofilm may be nonpathogenic but is capable of causing inflammation and bacterial dissemination depending on the host response. A tipping of the balance from colonization to overt biofilm mediated infection is a significant concern in the management of chronic wounds.1, 3
The use of animal models to investigate host–microbial interactions in human skin is limited by the intrinsic differences between the animal and human skin.4 Primary cultures of foreskin keratinocytes or immortalized keratinocyte cell lines are alternate in vitro methods to study the epithelial response to the skin flora.5 Potential changes in the cellular genetic make‐up subsequent to immortalization and the limited use of exfoliated surface squames are significant hurdles that compromise the applicability of these sources.
The human skin and oral mucous membrane share several structural and functional characteristics such as the lining by stratified squamous epithelium and the ability to respond to microbes via conserved pattern recognition receptors.5, 6, 7 Furthermore both skin and the oral epithelium undergo physiological desquamation as a measure to maintain the thickness and integrity of the physical barrier. However, a major distinction is the exfoliation of high percentage of nucleated epithelial cells from the mucosa as compared to the skin. The exfoliated epithelial cells from the oral mucosa constitute a significant cellular component in the saliva.5, 8
Previously others and we have shown that the epithelial cells derived from human saliva are viable and capable of responding to microbial products in vitro.4, 9 The objective of this study is to determine the applicability of salivary epithelial cells (SEC) as bio‐specimen to investigate the immune response to mono or dual species biofilm consisting of Staphylococcus epidermidis and Staphylococcus aureus bacteria representing commensal and transient pathogen of the skin flora, respectively. Our data suggest that the SEC secrete increased inflammatory cytokines such as IFN‐γ and IL‐12 in response to S. aureus biofilm as compared to S. epidermidis biofilm consistent with similar observations in primary epidermal keratinocytes.10, 11
Material and Methods
Biofilm preparation: Briefly, frozen stocks of S. aureus (ATCC 25923) and S. epidermidis (ATCC 155) were grown on Columbia Blood Agar plates supplemented with 5% sheep blood (Becton, Dickinson and Company, Heidelberg, Germany) for 24 hours at 37°C. Colony Forming Units (CFU) were then incubated at 37°C in Tryptic Soy Broth (TSB; Difco laboratories, Detroit, MI, USA) supplemented with 0.5% glucose (Fisher Scientific, Fair Lawn, NJ, USA) for 18 hours. Aliquots of individual strain suspension or a combination of equal volume of the two strains was then incubated in fresh TSB/0.5% glucose until an optical density OD550 = 0.8 (approximately 105 CFU/mL) was reached. 12, 13 Single or dual‐species suspension (0.5 mL) was overlaid on 2.0 mL of TSB/0.5% glucose in 24‐well culture plates. A piece of 0.9 × 0.8 mm of sterile nitrocellulose membrane was inserted in each well and the plate incubated at 37°C sequentially with and without constant agitation for 90 minutes. The membranes with adherent biofilms were used for co‐culture experiments.
Isolation of oral epithelial cells from saliva
The saliva was collected from a single healthy volunteer (with no known oral/systemic disorders) by the drooling method after obtaining informed consent in accordance with the institutional review board at IUPUI.9 Saliva was centrifuged at 3,000 rpm for 10 minutes. The pellet obtained was washed in isotonic diluent (Hematronix Inc., Plano, TX, USA) added 2 drops of Zap‐o‐Globin lytic reagent (Beckman Coulter, Brea, CA, USA) and then incubated in keratinocyte growth medium (Genlantis Inc, San Diego, CA, USA) supplemented with 5% penicillin/streptomycin at 37°C. The culture medium was changed every two days until the formation of a confluent monolayer of epithelial cells. Absence of leukocytes and the purity of SEC was determined by staining for leucocyte specific CD45 and epithelial cell specific pan‐cytokeratin, respectively and assessed by flow cytometry (data not shown). The cultures of SEC showed fewer and fewer microbes with each passage as visualized by light microscope and Gram's staining.
Co‐culture of oral epithelial cells and biofilms: The number of viable cells was determined by trypan blue exclusion using Countess, an automated cell counter (Invitrogen, Life Technologies, Grand Island, NY, USA). Epithelial cell suspension at 104 cells/mL was cultured in keratinocyte growth medium at 37°C and 5% CO2 in 24 well plates for 24 hours. Subsequently the cells were co‐cultured with membrane bound mono or dual‐species biofilm at 105species/membrane for periods of 0, 15, 30, 45, 60, and 75 minutes. Epithelial cells exposed to sterile membranes (without biofilms) served as control. The culture supernatant and the cells collected at the end of each time point were stored at −80°C until further analysis.
Real‐time polymerase chain reaction (RT‐PCR): Total cellular RNA isolated from the SECs was reverse transcribed and specific genes were amplified by real‐time PCR on the ABI Prism 7000 (Perkin Elmer Applied Systems, Foster City, CA, USA) as described.9 Message for small proline rich protein (SPRR 2a), a gene abundantly expressed in stratified squamous epithelia was amplified as an internal control. The primers include; toll like receptor (TLR)‐2F: 5′‐GGCCAGCAAA TTACCTGTGT‐3′; TLR‐2R: 5′‐TTCTCCACCCAGTAGGCATC‐3′ and SPRR2aF: 5′‐AGTGC CAGCAGAAATATCCTCC‐3′, SPRR2aR: 5′‐GAACGAGGTGAGCCAAATATCC‐ 3′.14 The fold change in the gene normalized to that of SPRR was determined by the 2−ΔΔCt method with untreated controls as the reference samples.
Enzyme linked immunosorbent assay (ELISA): Cytokines in the culture supernatants were determined using Opt ELISA kits (BD Opt™, BD Biosciences, San Jose, CA, USA).14 Data are expressed as percent production of cytokines with respect to the SEC cultured alone.
Statistical analysis: Statistical difference in cytokines and TLR‐2 expression between the different treatments of SEC was determined by pair‐wise t‐test. p < 0.05 was considered significant.
Results
Nature of the biofilm
The bacterial species for biofilm formation were cultured on sterile nitrocellulose membrane. After 3 hours of mono‐species biofilm formation, counts of S. aureus and S. epidermidis were 3.93 × 104 ± 2.2 × 104 and 6.38 × 105 ± 6.37 × 105 CFU/mm2, respectively. In dual‐species biofilms, counts of total cells, S. aureus and S. epidermidis were 1.4 × 105 ± 6.0 × 104 , 1.93 × 104 ± 6.21 × 103 and 4.88 × 104 ± 2.53 × 104 CFU/mm2, respectively. (Figure 1)
Figure 1.
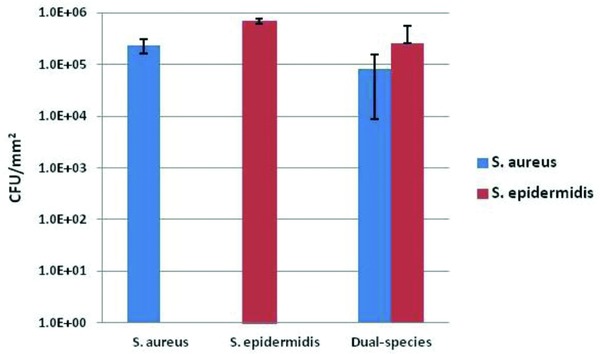
Biofilm grown on nitrocellulose membrane: Counts of viable bacteria as indicated on biofilms (CFU/mm2) after 3 hours of incubation (mean ± SD).
SEC express increased response to dual species biofilm
Human keratinocytes, oral and epidermal, have been shown to respond to microbes or their products by secreting pro‐inflammatory cytokines and chemokines.6 The functional potential of the purified SECs was evident from their ability to secrete cytokines in response to biofilm exposure. The concentration of IL‐6 in the supernatants of unstimulated SEC at 0,15, 30, 45, 60, and 75 minutes time points was 0.8, 2.6, 3.6, 5.0, and 7.1 ng/mL, respectively. The IL‐6 secretion exhibited a transient slight increase following exposure to mono or dual species biofilm followed by gradual decrease although no significant difference was observed between the supernatants collected at different time points (Figure 2A). The concentration of IL‐8 in the supernatants of unstimulated SEC at 0, 15, 30, 45, 60, and 75 minutes time points was 3.4, 3.6, 3.0, 3.0, 2.3, and 3.1 ng/mL, respectively. Stimulation of SEC with S. epidermidis biofilm or dual species biofilm mediated significantly higher IL‐8 secretion at one hour after exposure. IL‐8 concentration was equivalent in the culture supernatants of SEC stimulated with S. aureus biofilm at all time‐points assessed (Figure 2B). The concentration of IFN‐γ in the supernatants of unstimulated SEC at 0, 15, 30, 45, 60, and 75 minutes time points was 0.06, 0.2, 0.23, 0.96, 0.98, and 1.0 ng/mL, respectively. The percent production of IFN‐γ secretion was significantly higher in the culture supernatants of both mono and dual species biofilm at the later time points of 45, 60, and 75 minutes as compared to the supernatant collected at earlier time points. Interestingly, the cultures of SEC stimulated with S. aureus exhibited higher IFN‐γ secretion as compared with SEC cultured with S. epidermidis alone significantly at 60 and 75 minutes (Figure 2C). The concentration of IL‐12 in the supernatants of unstimulated SEC at 0, 15, 30, 45, 60, and 75 minutes time points was 4.0, 4.0, 4.3, 4.8, 4.6, and 5.0 ng/mL, respectively. The percent production of IL‐12 secretion was significantly higher in the culture supernatants of both mono and dual species biofilm at the 75 minutes time point as compared with the supernatant collected at the initiation of culture. The IL‐12 concentration was significantly higher in the 75 minutes supernatants of SEC cultures stimulated with S. aureus biofilm or dual species biofilm as compared with the cultures stimulated with S. epidermidis biofilm (Figure 2D).
Figure 2.
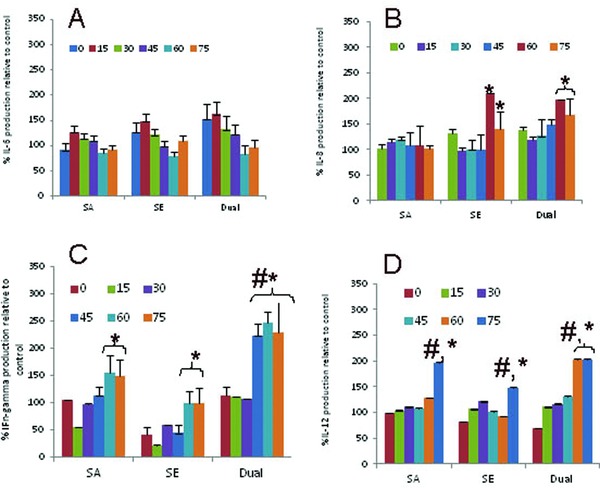
SEC secrete cytokines in response to cutaneous biofilm: Purified SEC at 105cells/mL were co‐cultured with mono (S. aureus/S. epidermidis) or dual species biofilm for the indicated time points as described in the Material and Methods section. Supernatant collected at the end of each time point was assessed for (A) IL‐6, (B) IL‐8, (C) INF‐γ, and (D) IL‐12. Data are presented as mean ± SD. * represent when compared to early time points of 0–45 minutes. # represent p < 0.05 of the cytokines in SEC cultures of S. aureus or dual species biofilm as compared to S. epidermidis mono species biofilm.
SEC upregulate TLR‐2 expression in response to cutaneous biofilm
Skin and oral keratinocytes recognize microbes via TLR that recognize specific pathogen associated molecular pattern shared by large groups of microorganisms.6, 15 Both S. aureus and S. epidermidis have been shown to induce signaling via TLR‐2 in keratinocytes and enhance protection against infection.10, 11 We observed that the TLR‐2 expression in SEC was highest at 30 minutes following stimulation with the S. aureus biofilm. There was an immediate upregulation of TLR‐2 in SEC following exposure to the S. epidermidis biofilm. Exposure to dual species biofilm induced a sustained upregulation of TLR‐2 in the SEC up to 60 minutes (Figure 3A and B).
Figure 3.
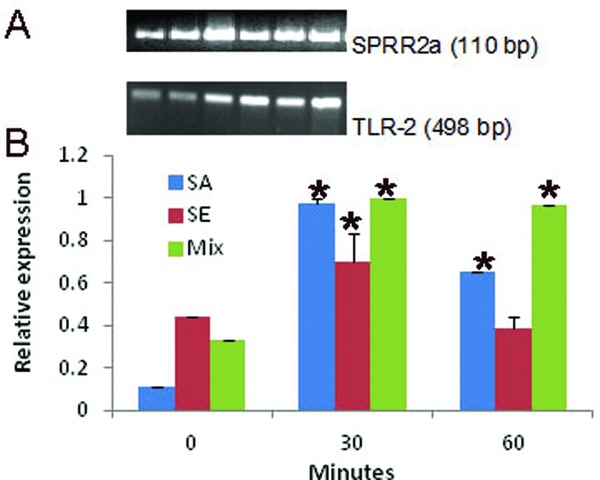
SECs upregulateTLR‐2 in response to cutaneous biofilm: Purified SEC at 104cells/mL were co‐cultured with mono (S. aureus/S. epidermidis) or dual species biofilm as described in the Material and Methods section. Total RNA isolated from the epithelial cells harvested at indicated time point was reverse transcribed. Equal amount of cDNA was amplified for SPRR2a and TLR‐2 by quantitative RT‐PCR using SYBR green master mix. (A) Gel electrophoresis of the PCR products. (B) Fold change of CD14, TLR‐2, and TLR‐4 was determined by the 2−ΔΔCt method. Each experiment was repeated three times. Data are presented as mean ± SD. * represents p < 0.05 when compared to unstimulated SEC.
Discussion
Chronic skin wounds are a major global health problem and considerable economic burden. Sustained inflammatory response and frequent microbial colonization of the wound bed contribute to wound chronicity. 16 Although characterization of host responses at an actual infection site is preferable, cellular heterogeneity is a potential complication.4 Here, we report for the first time the use of epithelial cells derived from human saliva as a viable biospecimen for investigating the host response to cutaneous biofilm.
Primary skin and oral keratinocytes have been shown to exhibit functional response to stimulation with heat killed S. aureus or S. epidermidis 10, 11, 17 or their products.18, 19 Both bacterial species are overrepresented in biofilms covering chronic wounds and skin burns. Infection with either species has also been implicated in severe oral mucositis.13, 20 Previously others and we have shown that the epithelial cells in the saliva respond to bacterial and fungal exposure by secreting cytokines.4, 14 In this study, we observed that the SECs exhibit a differential cytokine response to mono species biofilm with either S. epidermidis or S. aureus. As opposed to the commensal S. epidermidis, exposure to the transient pathogen S. aureus biofilm induced increased secretion of proinflammatory cytokines IFN‐γ and IL‐12. Importantly exposure of SEC to dual species biofilm induced a highly significant increase in the secretion of IFN‐γ that could suggest transition to inflammation/infection in vivo.
Both oral and cutaneous keratinocytes recognize and respond to the Gram+ streptococci via TLR‐2. Comparative analysis of gene expression using a human skin equivalent model showed that unlike the commensal S. epidermidis, colonization by S. aureus on intact skin stimulate increased expression of a diverse range of innate defense transcripts including TLR‐2.3 In primary epidermal keratinocytes S. aureus induces secretion of cytokines and thymic stromal lymphopoietin via TLR‐2 mediated signaling.11, 21 Recent evidence suggests that the commensal flora may coexist with the host in a mutualistic relationship and prohibit establishment of pathogenic microbes.22 For example priming of the skin by S. epidermidis, a ubiquitous commensal has been suggested to deter colonization by the transient pathogen S. aureus.23, 24 In addition, a unique lipoteichoic acid produced by S. epidermidis has been shown to inhibit uncontrolled inflammatory responses following cutaneous injury in a TLR‐2 dependent mechanism.24 We observed that the SEC upregulated TLR‐2 expression almost immediately following exposure to the commensal S. epidermidis. However further increase was modest as compared with the striking increase in the TLR‐2 expression at 30 minutes following exposure to the transient colonizer, the S. aureus. Sustained TLR‐2 increase in the SEC exposed to dual species biofilm suggests that the response to potential pathogen such as S. aureus supersedes the response to the commensal. The differential response of the SEC to the commensal versus transient colonizer is consistent with the suggestion that the host response is tuned to allow nonaggressive type to survive in the normal environment and the more aggressive type to evoke a protective response.3
Conclusion
Although our study did not include a direct comparison between the skin keratinocytes and the SEC or a comparative analysis of response to sessile versus planktonic bacteria, extrapolation of our data with the published literature on cutaneous epithelial responses suggest that the two cell systems function similarly and support the potential application of SEC as viable alternative model to investigate the host microbe response at the epidermal interphase. Furthermore, the SEC can serve as highly relevant personalized biospecimens for evaluating individualized response to biofilms.
Disclosures
The authors do not have conflicts of interest for the materials used in this study.
Acknowledgments
The study was supported partially by the State of Sao Paulo Research Foundation grant to Thais Negirini.
References
- 1. Chiller K, Selkin BA, Murakawa GJ. Skin microflora and bacterial infections of the skin. The Journal of Investigative Dermatology. Symposium Proceedings/the Society for Investigative Dermatology, Inc. [and] European Society for Dermatological Research. 2001; 6(3): 170–174. [DOI] [PubMed] [Google Scholar]
- 2. Kirketerp‐Møller K, Zulkowski K, Garth J. Chronic wound colonization, infection, and biofilms In: Bjarnsholt Th, Jensen PO, Moser C, Høiby N, eds. Biofilm Infections. New York: Springer Science, Business Media; 2011: 11–24. [Google Scholar]
- 3. Vlassova N, Han A, Zenilman JM, James G, Lazarus GS. New horizons for cutaneous microbiology: the role of biofilms in dermatological disease. Br J Dermatol. 2011; 165(4): 751–759. [DOI] [PubMed] [Google Scholar]
- 4. Lilly EA, Leigh JE, SH Joseph, Fidel PL, Jr . Candida‐induced oral epithelial cell responses. Mycopathologia. 2006; 162(1): 25–32. [DOI] [PubMed] [Google Scholar]
- 5. Liu J, Bian Z, Kuijpers‐Jagtman AM, Von den Hoff JW. Skin and oral mucosa equivalents: construction and performance. Orthodontics Craniofacial Res. 2010; 13(1): 11–20. [DOI] [PubMed] [Google Scholar]
- 6. Uehara A, Fujimoto Y, Fukase K, Takada H. Various human epithelial cells express functional Toll‐like receptors, NOD1 and NOD2 to produce anti‐microbial peptides, but not proinflammatory cytokines. Mol Immunol. 2007; 44(12): 3100–3111. [DOI] [PubMed] [Google Scholar]
- 7. Wertz P, Swartzendruber D, CA S. Regional variation in the structure and permeability of oral mucosa and skin. Adv Drug Deliv Rev. 1993. (12): 1–12. [Google Scholar]
- 8. Rowat JS, Squier CA. Rates of epithelial cell proliferation in the oral mucosa and skin of the Tamarin monkey (Saguinus fuscicollis). J Dent Res. 1986; 65(11): 1326–1331. [DOI] [PubMed] [Google Scholar]
- 9. Janardhanam SB, Prakasam S, Swaminathan VT, Kodumudi KN, Zunt SL, Srinivasan M. Differential expression of TLR‐2 and TLR‐4 in the epithelial cells in oral lichen planus. Arch Oral Biol. 2012; 57(5): 495–502. [DOI] [PubMed] [Google Scholar]
- 10. Lai Y, Cogen AL, Radek KA, Park HJ, Macleod DT, Leichtle A, Ryan AF, Di Nardo A, Gallo RL. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J Invest Dermatol. 2010; 130(9): 2211–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Niebuhr M, Baumert K, Werfel T. TLR‐2‐mediated cytokine and chemokine secretion in human keratinocytes. Exp Dermatol. 2010; 19(10): 873–877. [DOI] [PubMed] [Google Scholar]
- 12. Costerton JW. Introduction to biofilm. Int J Antimicrob Agents. 1999; 11(3–4): 217–221; discussion 237–219. [DOI] [PubMed] [Google Scholar]
- 13. Holland DB, Bojar RA, Jeremy AH, Ingham E, Holland KT. Microbial colonization of an in vitro model of a tissue engineered human skin equivalent—a novel approach. FEMS Microbiol. Lett. 2008; 279(1): 110–115. [DOI] [PubMed] [Google Scholar]
- 14. Srinivasan M, Kodumudi KN, Zunt SL. Soluble CD14 and toll‐like receptor‐2 are potential salivary biomarkers for oral lichen planus and burning mouth syndrome. Clin Immunol. 2008; 126(1): 31–37. [DOI] [PubMed] [Google Scholar]
- 15. Sugawara Y, Uehara A, Fujimoto Y, Kusumoto S, Fukase K, Shibata K, Sugawara S, Sasano T, Takada H. Toll‐like receptors, NOD1, and NOD2 in oral epithelial cells. J Dent Res. 2006; 85(6): 524–529 [DOI] [PubMed] [Google Scholar]
- 16. Acosta JB, del Barco DG, Vera DC, Savigne W, Lopez‐Saura P, Guillen Nieto G, Schultz GS. The pro‐inflammatory environment in recalcitrant diabetic foot wounds. Int Wound J. 2008; 5(4): 530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sasaki T, Kano R, Sato H, Nakamura Y, Watanabe S, Hasegawa A. Effects of staphylococci on cytokine production from human keratinocytes. Br J Dermatol. 2003; 148(1): 46–50. [DOI] [PubMed] [Google Scholar]
- 18. Chung WO, Dale BA. Innate immune response of oral and foreskin keratinocytes: utilization of different signaling pathways by various bacterial species. Infect Immunol. 2004; 72(1): 352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Uehara A, Sugawara S, Takada H. Priming of human oral epithelial cells by interferon‐gamma to secrete cytokines in response to lipopolysaccharides, lipoteichoic acids and peptidoglycans. J Med Microbiol. 2002; 51(8): 626–634. [DOI] [PubMed] [Google Scholar]
- 20. Smith AJ, Jackson MS, Bagg J. The ecology of Staphylococcus species in the oral cavity. J Med Microbiol. 2001; 50(11): 940–946. [DOI] [PubMed] [Google Scholar]
- 21. Vu AT, Baba T, Chen X, Le TA, Kinoshita H, Xie Y, Kamijo S, Hiramatsu K, Ikeda S, Ogawa H, et al. Staphylococcus aureus membrane and diacylated lipopeptide induce thymic stromal lymphopoietin in keratinocytes through the Toll‐like receptor 2‐Toll‐like receptor 6 pathway. J Allergy Clin Immunol. 2010; 126(5): 985–993, 993 e981–983. [DOI] [PubMed] [Google Scholar]
- 22. Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, et al. Compartmentalized control of skin immunity by resident commensals. Science. 2012; 337(6098): 1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011; 9(4): 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gallo RL, Nakatsuji T. Microbial symbiosis with the innate immune defense system of the skin. J Invest Dermatol. 2011; 131(10): 1974–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


