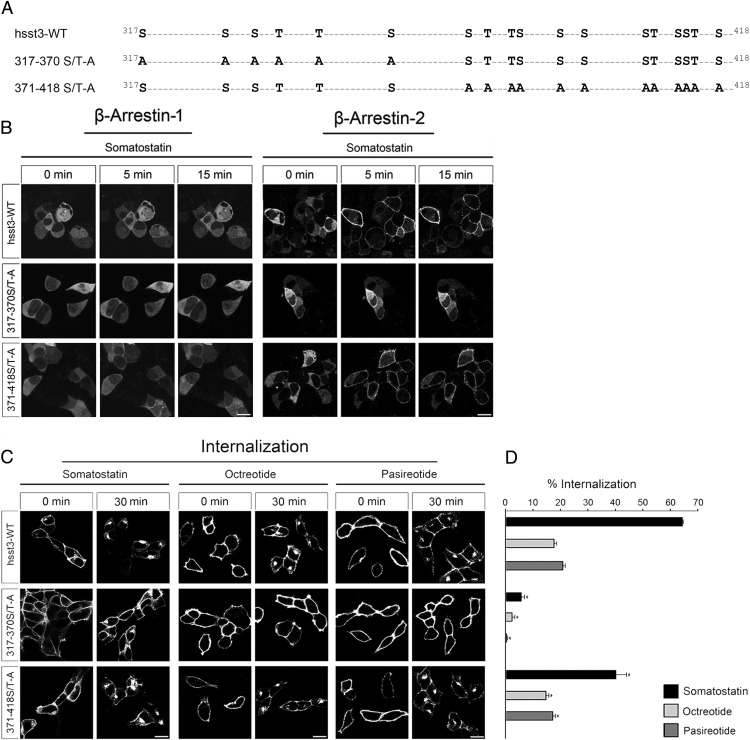
Figure 1. Construction of phosphorylation-deficient sst3 receptor mutants.
A, Schematic representation of the human sst3 receptor indicating all serine and threonine sites within the C-terminal tail (317–418). Phosphate acceptor sites mutated to alanine in the 317–370 S/T-A, and 371–418 S/T-A mutants are marked in bold. B, HEK293 cells were transiently transfected with either wild-type hsst3, 317–370 S/T-A or 371–418 S/T-A and β-arrestin-2-EGFP or β-arrestin-1-EGFP. The distribution of β-arrestin-2 and β-arrestin-1 were visualized sequentially in the same live cells before (0 min) and after (5 and 15 min) the addition of 1μM SS-14 to the culture medium. Shown are representative images from 1 of 4 independent experiments performed in duplicate. Scale bar, 15 μm. C, HEK293 cells stably expressing wild-type hsst3, 317–370 S/T-A, or 371–418 S/T-A were treated with 1μM somatostatin, octreotide, or pasireotide for 30 minutes. Cells were then fixed, stained with the anti-HA antibody, and examined by confocal microscopy. Shown are representative images from 1 of at least 3 independent experiments performed in duplicate. Scale bar, 15 μm. D, HEK293 cells stably expressing wild-type or mutant hsst3 receptors were treated for 30 minutes with 1μM agonist. Receptor sequestration was measured by surface ELISA. Data represent receptor internalization in agonist-treated cells as compared with sister cultures receiving 30 minutes of vehicle (100%). Data are presented as mean ± SEM from at least 4 independent experiments performed in quadruplicate. Results were analyzed by unpaired t test vs wild type receiving 30 minutes of vehicle (*, P < .05).