Abstract
Diabetes is characterized by a loss and dysfunction of the β-cell. Glucagon-like peptide 1 receptor (GLP-1R) signaling plays an important role in β-cell survival and function. It is meaningful to identify promising agents from natural products which might activate GLP-1R signaling. In this study, puerarin, a diet isoflavone, was evaluated its beneficial effects on β-cell survival and GLP-1R pathway. We showed that puerarin reduced the body weight gain, normalized blood glucose, and improved glucose tolerance in high-fat diet-induced and db/db diabetic mice. Most importantly, increased β-cell mass and β-cell proliferation but decreased β-cell apoptosis were observed in puerarin-treated diabetic mice as examined by immunostaining of mice pancreatic sections. The protective effect of puerarin on β-cell survival was confirmed in isolated mouse islets treated with high glucose. Further mechanism studies showed that the circulating level of GLP-1 in mice was unaffected by puerarin. However, puerarin enhanced GLP-1R signaling by up-regulating expressions of GLP-1R and pancreatic and duodenal homeobox 1, which subsequently led to protein kinase B (Akt) activation but forkhead box O1 inactivation, and promoted β-cell survival. The protective effect of puerarin was remarkably suppressed by Exendin(9–39), an antagonist of GLP-1R. Our study demonstrated puerarin improved glucose homeostasis in obese diabetic mice and identified a novel role of puerarin in protecting β-cell survival by mechanisms involving activation of GLP-1R signaling and downstream targets.
Loss of β-cell and the impaired function of β-cell might be the important pathogenesis of diabetes. The underlying mechanisms of β-cell failure in type 2 diabetes mellitus (T2DM) are complex and still largely unknown. Lipo- and glucotoxicity, subclinical inflammation, oxidative stress, and endoplasmic reticulum (ER) stress may play an important role in these processes (1, 2). Increase the β-cell mass and restore the function of β-cell become potential strategies for diabetes treatment. The incretin hormone glucagon-like peptide 1 (GLP-1) is an important target for diabetes therapy because of its ability to potentiate glucose-stimulated insulin secretion (GSIS) as well as to promote β-cell proliferation and survival (3). GLP-1 receptor (GLP-1R) agonists and dipeptidyl peptidase-IV (DPP-4) inhibitors have been developed as new therapies based on these incretin effects.
In the treatment of metabolic syndrome, the traditional Chinese medicine is an excellent alternative and complementary medicine with a long history. Recently, some natural products have been shown effects on regulating GLP-1 level. For example, it was reported that a naturally derived agent, berberine, promoted GLP-1 secretion in streptozotocin (STZ)-induced diabetic rats (4). A latest study showed that the ethyl acetate fraction of radix of Acorus calamus L. lowered blood glucose levels in STZ-induced mice and db/db mice via elevated GLP-1 secretion and gcg mRNA level (5).
The popularity of dietary natural product supplements is increasing worldwide even in Western countries. Puerarin, a diet isoflavone, is found in a number of herbs, such as the root of Radix puerariae (kudzu root), which is a nutritious food ingredient in East Asia traditionally. In Traditional Chinese Medicine, R. puerariae has been used for diabetic treatment for hundreds of years. Studies have shown that antidiabetic effects of puerarin are mediated by antioxidative (6), modulating lipid metabolism (7), and improving insulin secretion and resistance (8). A recent publication reported that puerarin inhibited β-cell death in STZ-induced diabetic mice, and its effect was mediated by the phosphoinositide 3-kinase (PI3K)/(AKT), protein kinase B, pathway (9). However, impacts of puerarin on β-cell function and proliferation have not been intensively investigated so far. The underlying mechanisms involved need to be assessed further.
The main goal for diabetes therapy is to prevent depletion of existing β-cells, as well as promoting new β-cell formation. In the present study, high-fat diet (HFD)-induced and db/db diabetic mice were applied to evaluate the antidiabetic effect of puerarin. Here, we aimed to investigate the direct effects of puerarin on β-cell proliferation and apoptosis in condition of hyperglycemia in vivo and in vitro. Effects of puerarin on GLP-1R signaling were examined for the first time in our study.
Materials and Methods
Reagent
Puerarin (purity > 98%) was purchased from the National Institute for the Control of Pharmaceutical and Biological Products. Exendin-4 and Exendin(9–39) were from Sigma.
Animals
All animal experiments were conducted in accordance with Provisions and General Recommendation of Chinese Experimental Animals Administration Legislation and approved by the Research Animal Care Committee of Nanjing Medical University. All animals were housed in a temperature-controlled room with a 12-hour light, 12-hour dark cycle and were allowed free access to food and water during the course of experiments. Four-week-old male C57BL/6J mice (SLAC Laboratory Animals) were fed a HFD (with 60 kcal% fat, D12492; Research Diets) or normal chow diet. Puerarin solution was prepared in 0.5% CMC-Na and delivered by oral gavage at dosage of 150 mg/kg daily. Puerarin intervention (150 mg/kg) was initiated after 12 weeks of the HFD and continued for 35 days. Four-week-old male C57Bl/KsJ (BKS) mice and BKS.Cg-Dock7m +/+ Leprdb/JNju (db/db) mice were ordered from Model Animal Research Center of Nanjing University. Puerarin was given for 55 days in db/db mice. The control group was given vehicle. After 35 or 55 days of puerarin administration, ip glucose tolerance test (IPGTT) was performed, and blood samples and pancreas were collected for subsequent analysis. A total of 12 mice in each group were used.
Intraperitoneal glucose tolerance tests
Mice were fasted 12 hours overnight and injected ip with glucose at a dose of 2-mg/g body weight. Blood samples were obtained at time points 0, 30, 60, 90, and 120 minutes for glucose measurements using a glucometer (Accu-Chek Active; Roche, Inc).
Blood parameters
Blood samples were collected from random-fed mice and centrifuged at 3000 rpm for 10 minutes at 4°C, and the serum was frozen until analysis. Diprotin A (DPP-4 inhibitor) and aprotinin were added to each blood sample to final concentrations of 100μM and 85 μg/mL, respectively. Insulin was determined using a mouse insulin ELISA kit (Alpco). Total cholesterol and triglycerol were assessed by kit (Nanjing Jiancheng). Serum adiponectin and leptin were assessed by mouse ELISA kit (Boster). GLP-1 was measured using a mouse/rat specific GLP-1(7–36) ELISA assay (Phoenix). All procedures were conducted according to the manufacturer's instructions.
Analysis of β-cell mass
β-Cell mass was measured as previously described (10). In brief, pancreatic sections were stained with antimouse insulin antibody (ab7842; Abcam) and scanned by a Nikon MEA53200 (Nikon) microscope. The cross-sectional areas of pancreas and β-cells were determined by NIS-Elements software (Nikon). β-Cell mass/pancreas was estimated by the product of the relative cross-sectional area of β-cells per total tissue and the weight of the pancreas.
Mouse pancreatic islets isolation and culture
Mouse islets were isolated from C57BL/6J mice (SLAC Laboratory Animals) by common bile duct perfusion using collagenase type 4 (Worthington) as described previously (11) and cultured in RPMI 1640 containing 11.1 mmol/L glucose, 100-U/mL penicillin, 100-mg/mL streptomycin, and 10% fetal bovine serum (FBS) (Invitrogen). For treatment of islets, medium was changed to culture medium containing 33.3mM glucose, treated with dimethyl sulphoxide (DMSO) as control or with puerarin (50μM), or Exendin-4 (50nM), or Exendin(9–39) (200nM) for 3 days.
Min6 cell culture
Min6 cells were obtained from American Type Culture Collection and maintained in 5mM glucose DMEM, supplemented with 10% fetal bovine serum (FBS) (Invitrogen), 50 mmol/L β-mercaptoethanol, 100-U/mL penicillin, and 0.1-mg/mL streptomycin in 5% CO2 at 37°C.
Glucose-stimulated insulin secretion
For acute insulin release, islets were washed and preincubated (30 min) in Krebs-Ringer bicarbonate buffer (KRB) containing 2.8mM glucose. The KRB was then replaced by KRB containing 2.8mM glucose for 1 hour (basal), followed by additional 1 hour of incubation in KRB containing 16.7mM glucose (stimulated). For insulin content, cells were extracted with 0.18N HCl in 70% ethanol. Insulin was determined using a mouse insulin ELISA kit (Alpco).
Immunofluorescence staining
Pancreatic tissue and cultured mouse islets were processed as previously described (12). Sections were fixed with 4% paraformaldehyde followed by permeabilization with 0.5% Triton X-100. Four-micrometer paraffin sections of mouse pancreases were deparaffinized, rehydrated, antigen unmasking. Then islets or sections were incubated overnight at 4°C with antiinsulin (ab7842; Abcam), anti-Ki67 (MKI67) (BD Pharmingen), antibodies followed by fluorescein isothiocyanate (FITC)- or Cyanine 3 (Cy3)-conjugated secondary antibodies (Jackson ImmunoResearch). β-Cell apoptosis in pancreatic sections was analyzed by the transferase-mediated dUTP nick end labeling (TUNEL) staining kit (in red) (In Situ Cell Death Detection kit, TMR red; Roche Diagnostics). β-Cell apoptosis in cultured islets was analyzed by another TUNEL kit (in black) (In Situ Cell Death Detection kit, AP; Roche Diagnostics). Slides were mounted with Vectashield with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Labs). Ki67 and TUNEL staining were analyzed using an Nikon MEA53200 (Nikon) microscope.
Membrane protein extraction
Membrane and cytoplasmic extractions of Min6 cells were performed according to the instructions of Mem-PER Membrane Protein Extraction Reagent (89826; Pierce Biotechnology). The purity of fractions was detected by anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) for cytosolic and anti-Na/K ATPase for membrane extracts.
RNA extraction and RT-PCR
Total RNA was isolated from cultured mouse pancreatic islets as described (12). For quantitative analysis, Applied Biosystems StepOne Real-Time PCR system (Applied Biosystems) with a commercial kit (Power SYBR Green PCR Master Mix; Applied Biosystems) was used. Primers used were: Ins1 (5′-TTCTTCTACACACCCA-3′, 5′-CTAGTTGCAGTAGTTCT-3′), GLP-1R (5′-CCTGAGGAACAGCTCCTGTC-3′, 5′-GGATGCAAACAGGTTCAGGT-3′), Tubulin (5′-GTTGGCCAGGCTGGTGTCCAG-3′, 5′-CTGTGATGAGCTGCTCAG GGTGG-3′), pancreatic and duodenal homeobox 1 (Pdx-1) (5′-GAGGACCCGTACTGCCTACA-3′, 5′-CGGG GTCCCGCTACTACGTT-3′), and T-cell factor 7-like 2 (TCF7L2) (5′-CAGGGAAGAACAGGCAAAAT-3′, 5′-GGGGGAGGCGAGTCTAGTAA-3′).
Western blot analysis
Cultured islets were washed in PBS and lysed. Polyvinylidene fluoride (PVDF) membranes were incubated with rabbit antiactin (4967), rabbit anti-phospho-Akt (Ser473, 9271), rabbit anti-Akt (9272), rabbit anti-c-Caspase 3 (9661), rabbit anti-p-forkhead box O1 (Foxo1) (Ser256, 9461), rabbit anti-Foxo1 (9462), rabbit anti-GAPDH (2118) (all Cell Signaling), rabbit anti-GLP-1R (ab39072; Abcam), rabbit anti-Pdx-1 (ab47267; Abcam), and rabbit anti-Na/K ATPase (ab76020), followed by incubation with horseradish peroxidase-linked IgG peroxidase. The bands were visualized, and densities of the bands were analyzed using Tanon ChemImaging Systems.
Statistical analysis
Data are presented as mean ± SE and were analyzed by paired, Student's t test or by ANOVA with a Bonferroni correction for multiple group comparisons.
Results
Puerarin normalized hyperglycemia and improved glucose tolerance in db/db and HFD diabetic mice
Based on previous reports (7, 8), we tried 2 doses of puerarin (chemical structure shown in Figure 1A), which were 150 and 300 mg/kg in preliminary study. Both of them showed significant glucose-lowering effects as compared with vehicle (data not shown). In the present study, we chose the dose of 150 mg/kg·d for subsequent experiments.
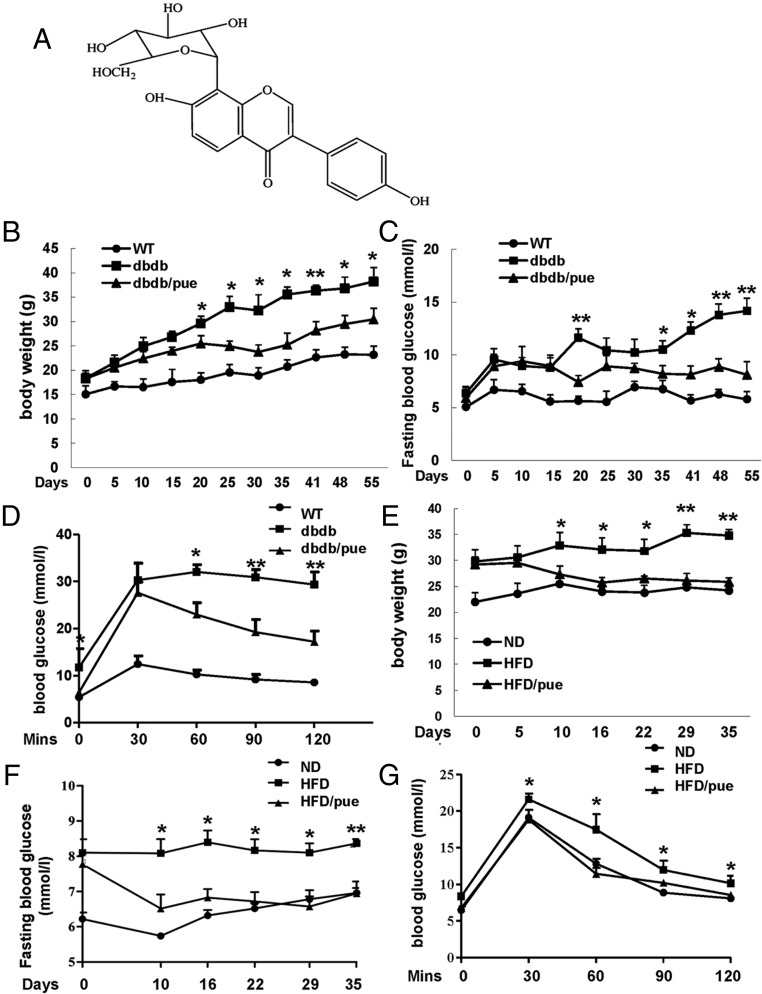
Figure 1. Puerarin normalized hyperglycemia and improved glucose tolerance in db/db and HFD diabetic mice.
A, Chemical structure of puerarin. B and C, Effects of puerarin on the body weights and fasting glucose levels in db/db group mice. D, After a 55-day treatment, IPGTTs were performed after a 12-hour fast with 2-mg/g BW of glucose in control, puerarin-, or vehicle-treated 12-week db/db mice. E and F, Effects of puerarin on the body weights and fasting glucose levels in HFD group mice. G, After a 35-day purarin treatment, IPGTTs were performed in control, puerarin-, or vehicle-treated 17-week HFD mice; *, P < .05; **, P < .01 puerarin to vehicle treated. Data are shown as mean ± SE, n = 12.
To assess the ability of puerarin to prevent progression of diabetes, we initiated puerarin treatment in 4-week db/db mice, which are prediabetic. The control group was given vehicle. During the whole 55-day experiment, body weight gain of db/db mice treated by puerarin was lower than that of the corresponding vehicle-treated db/db mice (Figure 1B). The vehicle db/db group developed to be diabetes at 6 weeks of age, and fasting blood glucose levels continued to increase over time (Figure 1C). In the first 15 days, puerarin did not strongly prevent progressing hyperglycemia in db/db mice. However, db/db mice were protected from developing such severe hyperglycemia by puerarin after 20 days of treatment, and this effect was maintained on subsequent days compared with vehicle-treated db/db mice. After the 55-day treatment, experiments of ip glucose challenge (IPGTT) were performed in these 12-week mice (Figure 1D). The response to IPGTT was seriously impaired in db/db mice which resulted in huge increased glucose levels compared with wild-type (WT) mice after glucose injection. Puerarin-treated db/db mice were kind of protected from such a severe glucose intolerance compared with vehicle-treated db/db mice. However, puerarin failed to prevent these mice to develop mild glucose intolerance, as compared with WT mice.
In parallel, another obese T2DM mouse model, 12-week HFD-induced diabetic mice were treated with puerarin for 35 days. Similarly, the body weight gain of HFD mice was deducted by puerarin significantly (Figure 1E). HFD feeding induced a marked increase in fasting blood glucose level in HFD mice compared with the normal diet (ND) mice (Figure 1F). Puerarin displayed hypoglycemic effect on HFD mice after 10 days of treatment compared with the vehicle, and this effect continued until the end of the experiment. In addition, the improvement of IPGTT in 17-week HFD mice with puerarin administration was observed as well (Figure 1G).
Puerarin increased β-cell mass and promoted β-cell survival without changing GLP-1 levels in db/db and HFD diabetic mice
Various natural products that increase plasma insulin levels and exert hypoglycemic effects in db/db mice have been reported (13–15). Here, we noticed the fasting serum insulin levels in 12-week db/db mice were decreased compared with WT mice, whereas puerarin significantly elevated the insulin levels in db/db mice compared with vehicle (Figure 2A). Similar increase of insulin level was observed in puerarin-treated 17-week HFD mice (Figure 2B).
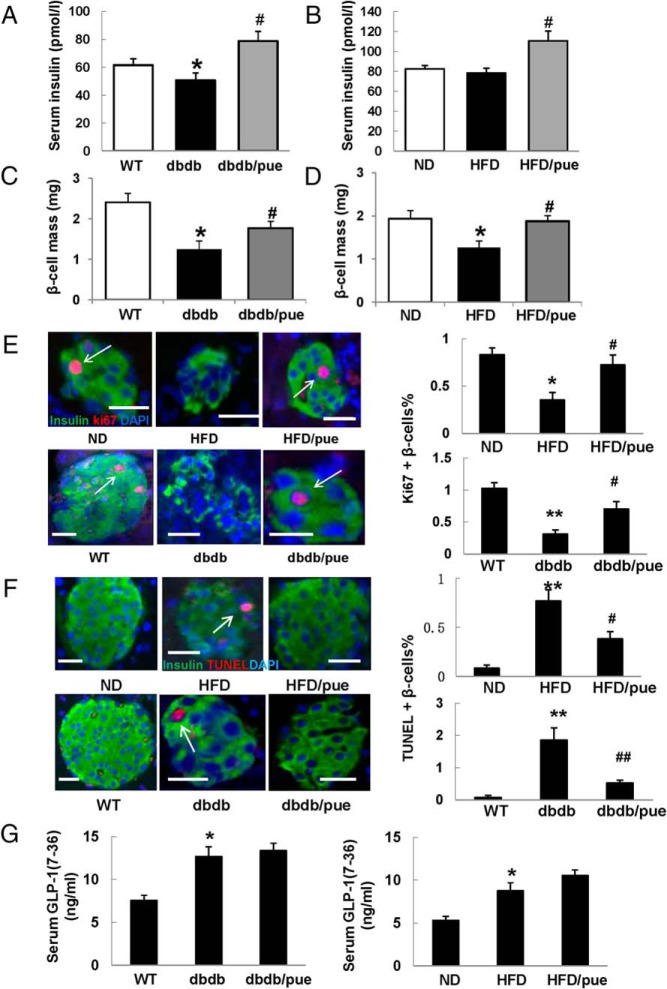
Figure 2. Puerarin increased β-cell mass and promoted β-cell survival.
After 35 or 55 days of puerarin administration, blood samples and pancreas of mice were collected for subsequent analysis. A and B, Serum insulin levels of db/db and HFD mice were measured by ELISA assay. Data are shown as mean ± SE. C and D, β-Cell mass as product of pancreas mass and insulin-positive area divided by section area. Six consecutive sections from each pancreas (9 mice per group) were used for β-cell mass measurements; n = 9; *, P < .05 db/db to WT or HFD to ND; #, P < .05 puerarin to vehicle. E, Proliferation of β-cell was measured in mice pancreatic sections by triple staining for Ki67 in red (indicated by white arrows), insulin in green, and DAPI in blue. F, Triple staining for TUNEL in red, insulin in green, and DAPI in blue was performed in mice pancreatic sections. Values are representative of 4 slides spanning the whole pancreas of each mouse and 6 mice per group. Data are shown as mean ± SE (n = 6); *, P < .05; **, P < .01 db/db to WT or HFD to ND; #, P < .05; ##, P < .01, puerarin to vehicle. Scale bars, 20 μm. G, Serum GLP-1 levels of db/db and HFD mice were detected by GLP-1(7–36) ELISA assay. Data are shown as mean ± SE; n = 9; *, P < .05 db/db to WT or HFD to ND.
Immunostaining for β-cells in pancreatic sections by insulin showed a significant reduction of β-cell mass in 12-week db/db and 17-week HFD mice vehicle treated (Figure 2, C and D). In contrast, the puerarin-treated group exhibited induction of β-cell mass (1.4-fold increase in db/db mice and 1.5-fold increase in HFD, compared with corresponding vehicle-treated mice), which may partially account for the increase of insulin level as we observed.
The increase in β-cell mass induced by puerarin raised the possibility that this compound may stimulate β-cell proliferation or prevent depletion of existing β-cells. Next, we investigated the impact of puerarin on β-cell proliferation by Ki67/insulin immunostaining in pancreatic sections (Figure 2E). Positive Ki67 staining of β-cells was observed in pancreatic sections of ND and WT mice but was rarely detected in HFD and db/db mice. In contrast, positive Ki67 staining was found in HFD and db/db mice treated with puerarin (2.05-fold increase in HFD and 2.25-fold increase in db/db mice, compared with vehicle, respectively).
In addition, we examined β-cell apoptosis by TUNEL/insulin immunostaining in pancreatic sections as well (Figure 2F). TUNEL-positive β-cell was barely found in sections from both ND groups and from WT group. Only 0.1% and 0.07% of β-cells were detected to be TUNEL-positive. The β-cell apoptosis was strongly increased in HFD and db/db mice (0.76% and 1.85%, respectively), which could be significantly suppressed by puerarin (2.1-fold reduction in HFD and 3.5-fold reduction in db/db mice, respectively, compared with vehicle).
These observations suggest that puerarin might exert a glucose lowering effect by protecting β-cells from damages caused by hyperglycemia. The incretin hormone GLP-1 is a key regulator to maintain glucose homeostasis by increasing insulin secretion as well as promoting β-cell survival (3). We wondered whether the effect of puerarin on β-cell survival is resulted from increase of GLP-1 level. As shown in Figure 2G, the circulating levels of active GLP-1 in db/db mice and HFD mice were increased compared with WT or ND mice, respectively. However, puerarin failed to elevate GLP-1 levels significantly despite its potential β-cell protective effects.
Puerarin improved β-cell function and up-regulating GLP-1R expression
GLP-1 acts through the cognate G protein-coupled receptors GLP-1R, which are expressed in several tissues, including pancreatic islets (16). In next study, impacts of puerarin on expression of GLP-1R and activation of the downstream targets were detected in isolated mouse islets cultured in high-glucose condition to mimic the hyperglycemia in vivo.
Results of RT-PCR from islets displayed that GLP-1R mRNA level was decreased by high glucose, whereas it was remarkably increased up to 7.5-fold by puerarin compared with the DMSO treatment (Figure 3A). Pdx-1 is a transcription factor that critically regulates β-cell specific genes such as insulin, glucose transporter 2 expressions and multiple aspects of β-cell function and survival. mRNA levels of Pdx-1 and Ins1 were induced by puerarin to 4.8- and 3.1-fold, respectively, compared with the DMSO treatment (Figure 3A). Interesting, here, we found that puerarin could up-regulate TCF7L2 mRNA level with 3.4-fold (to the DMSO) (Figure 3A). TCF7L2 is an important transcription factor of wingless-type MMTV integration site (Wnt)/β-catenin signaling, which is a key modulator for β-cell survival and regeneration (17, 18).
Figure 3. Puerarin improved β-cell function and up-regulating GLP-1R expression.
A, Real-time PCR analysis from mRNA isolated from the islets cultured in 33.3mM glucose with/without puerarin. Results were normalized to tubulin. Data are shown as mean ± SE from 3 independent experiments. B, GSIS assay was performed in treated mouse islets with/without puerarin. Stimulatory index denotes the amount of stimulated divided by the amount of basal insulin secretion. Data are shown as mean ± SE from 3 independent experiments. C, Representative Western blottings from the islets cultured in 33.3mM glucose with different treatments. D, Western blot analysis of the GLP-1R cellular distribution in Min6 cells after treatment with puerarin. The densitometric analyses of 3 independent experiments are shown; *, P < .05; **, P < .01, 33.3 to 11.1 or 5; #, P < .05, puerarin to DMSO.
Meanwhile, GSIS assay revealed that the insulin secretion of islets was strongly impaired by 33.3mM glucose compared with 11.1mM glucose incubations. Puerarin, however, induced a 1.8-fold increase in the stimulatory index, as compared with DMSO treated (Figure 3B).
Simultaneously, we measured the GLP-1R and Pdx-1 expressions in treated islets by Western blotting (Figure 3C). High glucose decreased GLP-1R as well as Pdx-1 expressions while inducing caspase3 cleavage, which triggered to β-cell apoptosis. Confirmed with RT-PCR results, puerarin significantly restored the GLP-1R and Pdx-1 expressions but reduced caspase3 cleavage to preserve β-cell survival.
A recent report showed that chronic hyperglycemia could lead to the loss of the GLP-1R from the cell surface and an impairment of GLP-1R signaling (19). Here, we examined the GLP-1R expression at the plasma membrane and cytoplasm, respectively, in Min6 cells with 33.3mM glucose treatment (Figure 3D). GLP-1R level at the membrane and the membrane to cytosolic ratio of GLP-1R were remarkably deducted by high glucose. Again, puerarin up-regulated the expression of GLP-1R and recovered the membrane distribution of GLP-1R.
Puerarin promoted β-cell function and survival via GLP-1R signaling activation
Antiapoptotic mechanisms of GLP-1 are mediated through activation of Akt (20). Akt is a key molecule of PI3K/Akt pathway that regulates glucose metabolism and β-cell survival. In view of the fact that puerarin could up-regulate GLP-1R expression, we detected activation of Akt and Foxo1, which are downstream targets of GLP-1R signaling (Figure 4A). As a member of the forkhead transcription factor family, the action of Foxo1 is suppressed when phosphorylated by Akt (21). As expected, puerarin activated Akt but inactivated Foxo1 significantly by inducing Akt and Foxo1 phosphorylations in islets treated by high glucose compared with DMSO control. Interesting, Exendin(9–39), an antagonist of GLP-1R, showed the strong ability to inhibit the Akt activation and Foxo1 inactivation induced by puerarin.
Figure 4. Puerarin promoted β-cell function and survival depended on GLP-1R signaling activation.
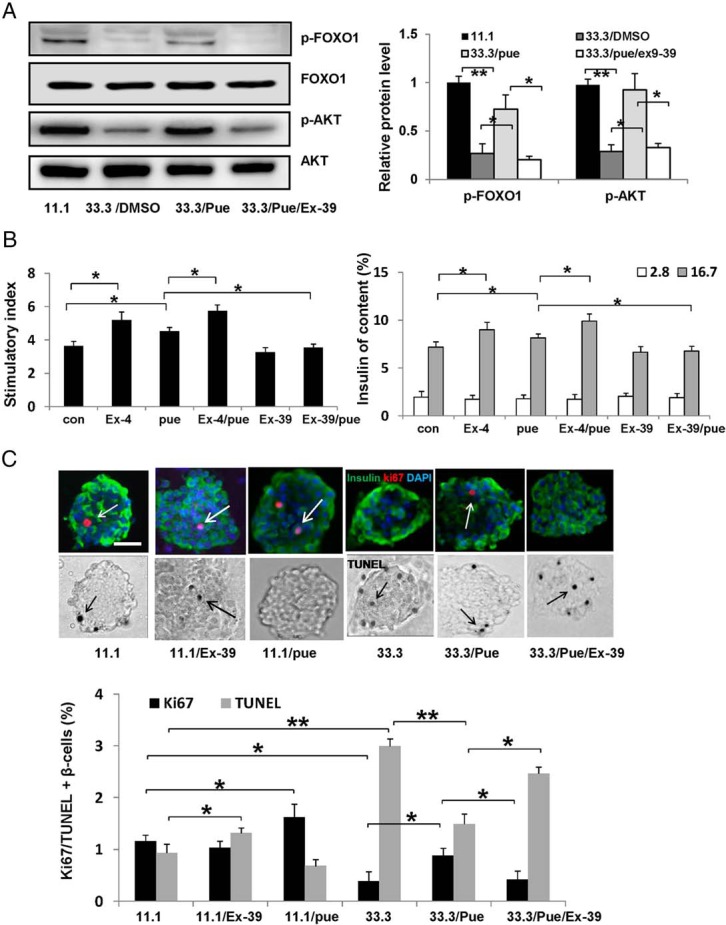
A, Representative Western blottings to show the activation of GLP-1R signaling in the islets cultured in 33.3mM glucose with different treatments, puerarin (50μM), or Exendin(9–39) (Ex-39) (200nM), DMSO as control. The densitometric analyses of 3 independent experiments are shown; *, P < .05; **, P < .01. B, GSIS assay was performed in treated mouse islets with different treatments, puerarin (50μM), or Exendin-4 (Ex-4) (50nM), or Ex-39 (200nM), DMSO as control. Stimulatory index denotes the amount of stimulated divided by the amount of basal insulin secretion. Data are shown as mean ± SE from 3 independent experiments. C, Isolated mouse islets were exposed to different conditions for 3 days. Proliferation was measured by the Ki67 staining (in red, indicated by white arrows), and apoptosis by the TUNEL assay stained in black (indicated by black arrows). Islets were triple stained for insulin in green and counterstained for DAPI in blue. Scale bars, 20 μm. Results are expressed as mean ± SE of the percentage of Ki67-positive or TUNEL-positive β-cells; *, P < .05; **, P < .01.
To explore the role of GLP-1R signaling in effects of puerarin, we performed GSIS assay in mouse islets with different treatments. As shown in Figure 4B, the insulin stimulatory effect of Exendin-4, a well-known agonist of GLP-1R, was significantly enhanced by puerarin. However, the insulin stimulatory effect of puerarin was inhibited by Exendin(9–39). Exendin(9–39) itself alone displayed an impairment of insulin secretion compared with control, but there was no significant difference, which was consistent with other report (22).
Further, cultured isolated mouse islets were applied to confirm the protective effects of puerarin on β-cell survival detected by immunostaining with Ki67 and TUNEL (Figure 4C). In normal condition (11.1mM glucose), puerarin itself showed an ability to stimulate the β-cell proliferation (1.39-fold increased by puerarin compared with DMSO treated). Elevated glucose level (33.3mM) reduced β-cell proliferation while inducing β-cell apoptosis, compared with control (11.1mM glucose). On the contrary, islets treated with puerarin were protected against the deleterious effects of high glucose. Proliferation of β-cell was 2.3-fold increased by puerarin compared with DMSO treated, whereas apoptosis was 2.1-fold decreased accordingly. Moreover, the protective effects of puerarin on β-cell survival were significantly suppressed by Exendin(9–39) (2.1-fold decrease in proliferation while 1.66-fold increase in apoptosis). Here, we noticed that Exendin(9–39) had no significant effect on β-cell proliferation but rather increased β-cell apoptosis.
Puerarin presented beneficial lipid metabolic effects in db/db and HFD diabetic mice
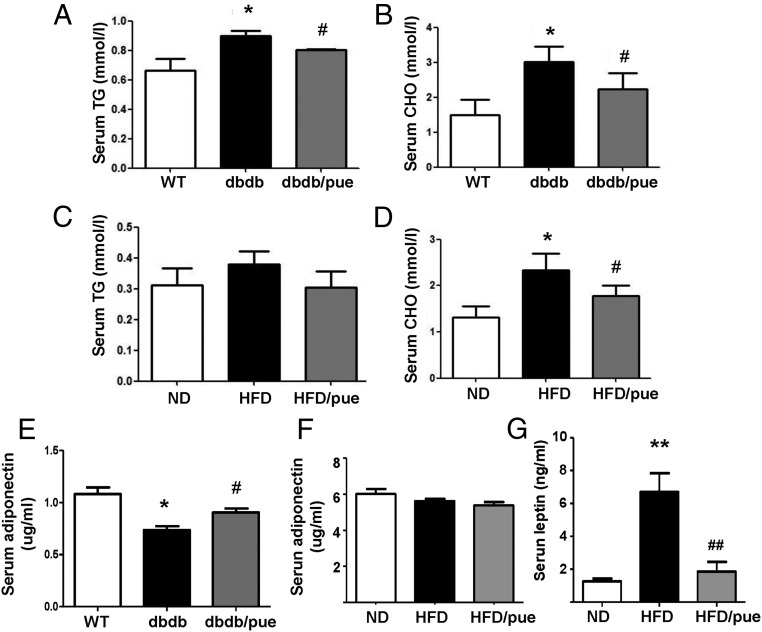
Hyperglycemia and insulin resistance give rise to diabetic dyslipidemia in T2DM. It is well known that GLP-1 could also modulate the lipid metabolism as a key regulator (23). HFD and db/db mice are both obese diabetic animal models with elevated total cholesterol and triglyceride contents. We found that puerarin decreased cholesterol and triglyceride levels significantly in db/db mice (Figure 5, A and B). In HFD mice, puerarin reduced total cholesterol significantly but had no remarkable effect on triglyceride levels (Figure 5, C and D). Recent studies suggest that adipokines can play a beneficial role in various metabolic diseases, especially in T2DM. Two important adipokines, adiponectin and leptin were measured as well. Puerarin up-regulated circulating adiponectin level in db/db mice, which was decreased in vehicle db/db group compared with WT mice (Figure 5E), whereas no significant change of adiponectin was found in HFD mice (Figure 5F). However, the induction of leptin in HFD mice was significantly reduced by puerarin compared with vehicle group (Figure 5G). These data indicated that the beneficial lipid metabolic effects of puerarin in HFD and db/db mice might be associated with the activation of GLP-1R pathway by puerarin.
Figure 5. Puerarin presented beneficial lipid metabolic effects in HFD and db/db mice.
A–D, Puerarin modulated serum total CHO (cholesterol), TG (triglyceride) levels of db/db and HFD mice. E–G, Beneficial effects of puerarin on serum leptin and adiponectin levels of db/db and HFD mice. Data are shown as mean ± SE, n = 9; *, P < .05; **, P < .01 db/db to WT or HFD to ND; #, P < .05; ##, P < .01 puerarin to vehicle.
Discussion
Healthy β-cells can compensate for insulin resistance by increasing in number and functional output. Pancreatic β-cell replication plays a primary role in keeping β-cell mass (24). However, β-cell mass is decreased in patients with diabetes mellitus, suggesting a primary role for β-cell depletion in the pathogenesis of T2DM (25). In this study, increased β-cell apoptosis and decreased β-cell proliferation were obtained in 17-week HFD mice and 12-week db/db mice, which might be resulted from long-term high-glucose toxicity and lipotoxicity. Significant induction of β-cell apoptosis in db/db mice was also reported by other study (26).
A variety of animal models of T1DM and T2DM have been used for diabetes-related researches, each with their own characteristics. STZ-induced type 1 diabetic mice were employed commonly in studies of puerarin antidiabetic effects (8, 9). However, STZ mice are not appropriate animal model for T2DM study. Few studies have assessed antidiabetic effects of puerarin in obese diabetic mice models so far. Growing evidence has shown obesity is a risk for T2DM, which might induce insulin resistance as well as impair β-cell function and survival (27). In the present study, we applied HFD mice and db/db mice to evaluate the puerarin antidiabetic effect, which are 2 commonly used obese diabetic mouse models for T2DM study (28). Four-week db/db mice start to display metabolic disorders and naturally develop diabetes by 6–8 weeks age. Severe depletion of the β-cells is observed in these mice with aging. As the diet-induced obesity mouse model, feeding a HFD in C57Bl/6 mice resulted in hyperinsulinemia and impaired glucose tolerance after 4 weeks, elevation of fasting glucose after 8 weeks, hyperglycemia, and loss of GSIS after 12 weeks of the HFD. β-Cell apoptosis is increased after 16 weeks of the HFD. Thus, HFD-induced diabetic mice and db/db mice applied in our study were nice models for β-cell survival study.
Herbal medicine as one of the most popular alternative medicines has a key role in the treatment of diabetes and other metabolic diseases in many developing countries. More than two-thirds of the active agents of drugs have relationship to natural sources (29). A large number of herbs and extracts have been shown effects on preventing metabolic disorders like diabetes and obesity with different mechanisms (30). Some natural components have functions on improving β-cell proliferation. For instance, genistein, a flavonoid in legumes and some herbal medicines, directly modulates pancreatic β-cell proliferation and function via activation of the cAMP/protein kinase A (PKA)-dependent ERK1/2 signaling pathway (31). Here, we demonstrated that puerarin could promote β-cell proliferation in HFD and db/db mice in vivo detected by Ki67/insulin costaining. Meanwhile, the β-cell apoptosis could be significantly suppressed by puerarin measured by TUNEL staining in mice pancreatic sections. More β-cell proliferation and less β-cell apoptosis contributed together to the increase of β-cell mass by puerarin in HFD and db/db mice.
The GLP-1R signaling controls the physiological response to GLP-1 and is currently a major target for the development of therapeutics owing to the broad range of potential beneficial effects in T2DM. These include promotion of glucose-dependent insulin secretion, increased insulin biosynthesis, preservation of β-cell mass, improved peripheral insulin sensitivity, and promotion of weight loss (32). The decrease of GLP-1R expression induced by high glucose was found in this study which was confirmed to our previous report (12). In line with our data, a decrease in GLP-1R and GIP-R has been observed in response to hyperglycemia, significant reductions occurring in islets from 90% pancreatectomized hyperglycemic rats as well as in isolated rat islets cultured in high glucose for 48 hours (33). Another study reported that the expressions of GLP-1R, gastric inhibitory polypeptide receptor (GIPR), and peroxisome proliferator-activated receptor α (PPARα) were down-regulated when INS-1 cells were treated with glucose, whereas their expressions were up-regulated when treated with metformin (34). Therefore, besides GLP-1R agonists and DPP-4 inhibitors, recovery the GLP-1R expression itself and enhance the GLP-1R signaling activation might be an alternative strategy for diabetic treatment.
The present study revealed that puerarin rescued the β-cell failure and promoted β-cell proliferation through up-regulating GLP-1R expression and activating its downstream target Akt, which led to inactivation of Foxo1 and caspase3 subsequently. Foxo1 acts as a transcription factor to inhibit Pdx-1 activity and mediate β-cell dysfunction and apoptosis. The increase of Pdx-1 by puerarin was obtain as well. The protective effect of puerarin was remarkably suppressed by Exendin(9–39), an antagonist of GLP-1R, which indicated that the function of puerarin is GLP-1R dependent. It is well known that mRNA is not a direct indication of protein level. Different regulation mechanisms (such as synthesis and degradation rates), acting on both the synthesized mRNA and the synthesized protein, affect the amount of the 2 molecules differentially (35). That is why the changes in protein expressions of GLP-1R and Pdx-1 shown in our study were not exactly correlated with changes in mRNA levels.
Interesting, here, we noticed that puerarin up-regulated the TCF7L2 expression as well. As a major transcription factor of Wnt signaling, studies have showed that Wnt/TCF7L2 pathway interacts with GLP-1 signaling. TCF7L2/β-catenin fosters synthesis of GLP-1 in intestinal L cells (36). Liu and Habener (37) reported that GLP-1 and Exendin 4 activated TCF7L2-dependent Wnt signaling to enhance β-cell proliferation. Wnt/TCF7L2 was able to improve β-cell turnover and function (38) (39). One new publication presented that TCF7L2 could positively regulate expressions of transcription factors like musculoaponeurotic fibrosarcoma oncogene family A and Pdx-1, which are crucial for β-cell regeneration (40). Here, we observed the increasing of TCF7L2 and Pdx-1 expressions induced by puerarin. These observations implied modulating effects of puerarin on Wnt signaling which need to be investigated in our future study.
In conclusion, we identified a novel role of puerarin in β-cell survival by mechanisms involving the activation of GLP-1R signaling. Our finding highlights the potential value of puerarin as a possible treatment of T2DM.
Acknowledgments
This work was supported by the European Foundation for the Study of Diabetes (EFSD), the Chinese Diabetes Society (CDS), and the Lilly Programme for Collaborative Research between China and Europe, Natural Science Foundation of China Grants 81102488 and 81370924, the Natural Science Foundation of Jiangsu Province Grant BK2011865, the Foundation for High-Level Talent in Six Areas of Jiangsu Province Grant 2015-YY010, and the Foundation of Jiangsu Province Administration of Traditional Chinese Medicine Grant LZ13066.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the European Foundation for the Study of Diabetes (EFSD), the Chinese Diabetes Society (CDS), and the Lilly Programme for Collaborative Research between China and Europe, Natural Science Foundation of China Grants 81102488 and 81370924, the Natural Science Foundation of Jiangsu Province Grant BK2011865, the Foundation for High-Level Talent in Six Areas of Jiangsu Province Grant 2015-YY010, and the Foundation of Jiangsu Province Administration of Traditional Chinese Medicine Grant LZ13066.
Footnotes
- Akt
- protein kinase B
- DPP-4
- dipeptidyl peptidase-IV
- DAPI
- 4′,6-diamidino-2-phenylindole
- DMSO
- dimethyl sulphoxide
- Foxo1
- forkhead box O1
- GLP-1
- glucagon-like peptide 1
- GLP-1R
- GLP-1 receptor
- GSIS
- glucose-stimulated insulin secretion
- HFD
- high-fat diet
- IPGTT
- ip glucose tolerance test
- Ki67
- MKI67
- KRB
- Krebs-Ringer bicarbonate buffer
- ND
- normal diet
- Pdx-1
- pancreatic and duodenal homeobox 1
- STZ
- streptozotocin
- TCF7L2
- T-cell factor 7-like 2
- T2DM
- type 2 diabetes
- TUNEL
- Transferase-mediated dUTP nick end labeling
- Wnt
- wingless-type MMTV integration site
- WT
- wild type.
References
- 1. Prentki M, Nolan CJ. Islet β cell failure in type 2 diabetes. J Clin Invest. 2006;116(7):1802–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H. Glucose toxicity in β-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes. 2003;52(3):581–587. [DOI] [PubMed] [Google Scholar]
- 3. Tosch W, Lanthaler K, Boote V, et al. . Molecular species of phosphatidylethanolamine from continuous cultures of Saccharomyces pastorianus syn. carlsbergensis strains. Yeast. 2006;23(2):75–82. [DOI] [PubMed] [Google Scholar]
- 4. Liu Q, Chen L, Hu L, Guo Y, Shen X. Small molecules from natural sources, targeting signaling pathways in diabetes. Biochim Biophys Acta. 2010;1799(10–12):854–865. [DOI] [PubMed] [Google Scholar]
- 5. Liu YX, Si MM, Lu W, et al. . Effects and molecular mechanisms of the antidiabetic fraction of Acorus calamus L. on GLP-1 expression and secretion in vivo and in vitro. J Ethnopharmacol. 2015;166:168–175. [DOI] [PubMed] [Google Scholar]
- 6. Xiong FL, Sun XH, Gan L, Yang XL, Xu HB. Puerarin protects rat pancreatic islets from damage by hydrogen peroxide. Eur J Pharmacol. 2006;529(1–3):1–7. [DOI] [PubMed] [Google Scholar]
- 7. Zhang W, Liu CQ, Wang PW, et al. . Puerarin improves insulin resistance and modulates adipokine expression in rats fed a high-fat diet. Eur J Pharmacol. 2010;649(1–3):398–402. [DOI] [PubMed] [Google Scholar]
- 8. Wu K, Liang T, Duan X, Xu L, Zhang K, Li R. Anti-diabetic effects of puerarin, isolated from Pueraria lobata (Willd.), on streptozotocin-diabetogenic mice through promoting insulin expression and ameliorating metabolic function. Food Chem Toxicol. 2013;60:341–347. [DOI] [PubMed] [Google Scholar]
- 9. Li Z, Shangguan Z, Liu Y, et al. . Puerarin protects pancreatic β-cell survival via PI3K/Akt signaling pathway. J Mol Endocrinol. 2014;53(1):71–79. [DOI] [PubMed] [Google Scholar]
- 10. Sauter NS, Schulthess FT, Galasso R, Castellani LW, Maedler K. The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology. 2008;149(5):2208–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shu L, Sauter NS, Schulthess FT, Matveyenko AV, Oberholzer J, Maedler K. Transcription factor 7-like 2 regulates β-cell survival and function in human pancreatic islets. Diabetes. 2008;57(3):645–653. [DOI] [PubMed] [Google Scholar]
- 12. Shu L, Matveyenko AV, Kerr-Conte J, Cho JH, McIntosh CH, Maedler K. Decreased TCF7L2 protein levels in type 2 diabetes mellitus correlate with downregulation of GIP- and GLP-1 receptors and impaired β-cell function. Hum Mol Genet. 2009;18(13):2388–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao Z, Choi J, Zhao C, Ma ZA. FTY720 normalizes hyperglycemia by stimulating β-cell in vivo regeneration in db/db mice through regulation of cyclin D3 and p57(KIP2). J Biol Chem. 2012;287(8):5562–5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Han SJ, Choi SE, Yi SA, et al. . β-Cell-protective effect of 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid as a glutamate dehydrogenase activator in db/db mice. J Endocrinol. 2012;212(3):307–315. [DOI] [PubMed] [Google Scholar]
- 15. Do GM, Jung UJ, Park HJ, et al. . Resveratrol ameliorates diabetes-related metabolic changes via activation of AMP-activated protein kinase and its downstream targets in db/db mice. Mol Nutr Food Res. 2012;56(8):1282–1291. [DOI] [PubMed] [Google Scholar]
- 16. Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology. 1996;137(7):2968–2978. [DOI] [PubMed] [Google Scholar]
- 17. Welters HJ, Kulkarni RN. Wnt signaling: relevance to β-cell biology and diabetes. Trends Endocrinol Metab. 2008;19(10):349–355. [DOI] [PubMed] [Google Scholar]
- 18. Jin T, Liu L. The Wnt signaling pathway effector TCF7L2 and type 2 diabetes mellitus. Mol Endocrinol. 2008;22(11):2383–2392. [DOI] [PubMed] [Google Scholar]
- 19. Rajan S, Dickson LM, Mathew E, et al. . Chronic hyperglycemia downregulates GLP-1 receptor signaling in pancreatic β-cells via protein kinase A. Mol Metab. 2015;4(4):265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li L, El-Kholy W, Rhodes CJ, Brubaker PL. Glucagon-like peptide-1 protects β cells from cytokine-induced apoptosis and necrosis: role of protein kinase B. Diabetologia. 2005;48(7):1339–1349. [DOI] [PubMed] [Google Scholar]
- 21. Kitamura T, Nakae J, Kitamura Y, et al. . The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic β cell growth. J Clin Invest. 2002;110(12):1839–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sathananthan M, Farrugia LP, Miles JM, et al. . Direct effects of exendin-(9,39) and GLP-1-(9,36)amide on insulin action, β-cell function, and glucose metabolism in nondiabetic subjects. Diabetes. 2013;62(8):2752–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patel VJ, Joharapurkar AA, Shah GB, Jain MR. Effect of GLP-1 based therapies on diabetic dyslipidemia. Curr Diabetes Rev. 2014;10(4):238–250. [DOI] [PubMed] [Google Scholar]
- 24. Baggio LL, Drucker DJ. Therapeutic approaches to preserve islet mass in type 2 diabetes. Annu Rev Med. 2006;57:265–281. [DOI] [PubMed] [Google Scholar]
- 25. Kitamura T. The role of FOXO1 in β-cell failure and type 2 diabetes mellitus. Nat Rev Endocrinol. 2013;9(10):615–623. [DOI] [PubMed] [Google Scholar]
- 26. Wang X, Liu Y, Yang Z, et al. . Glucose metabolism-related protein 1 (GMRP1) regulates pancreatic β cell proliferation and apoptosis via activation of Akt signalling pathway in rats and mice. Diabetologia. 2011;54(4):852–863. [DOI] [PubMed] [Google Scholar]
- 27. Niswender K. Diabetes and obesity: therapeutic targeting and risk reduction - a complex interplay. Diabetes Obes Metab. 2010;12(4):267–287. [DOI] [PubMed] [Google Scholar]
- 28. King AJ. The use of animal models in diabetes research. Br J Pharmacol. 2012;166(3):877–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75(3):311–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tabatabaei-Malazy O, Larijani B, Abdollahi M. Targeting metabolic disorders by natural products. J Diabetes Metab Disord. 2015;14:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fu Z, Zhang W, Zhen W, et al. . Genistein induces pancreatic β-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology. 2010;151(7):3026–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koole C, Pabreja K, Savage EE, et al. . Recent advances in understanding GLP-1R (glucagon-like peptide-1 receptor) function. Biochem Soc Trans. 2013;41(1):172–179. [DOI] [PubMed] [Google Scholar]
- 33. Xu G, Kaneto H, Laybutt DR, et al. . Downregulation of GLP-1 and GIP receptor expression by hyperglycemia: possible contribution to impaired incretin effects in diabetes. Diabetes. 2007;56(6):1551–1558. [DOI] [PubMed] [Google Scholar]
- 34. Pan QR, Li WH, Wang H, et al. . Glucose, metformin, and AICAR regulate the expression of G protein-coupled receptor members in INS-1 β cell. Horm Metab Res. 2009;41(11):799–804. [DOI] [PubMed] [Google Scholar]
- 35. de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C. Global signatures of protein and mRNA expression levels. Mol Biosyst. 2009;5(12):1512–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yi F, Brubaker PL, Jin T. TCF-4 mediates cell type-specific regulation of proglucagon gene expression by β-catenin and glycogen synthase kinase-3β. J Biol Chem. 2005;280(2):1457–1464. [DOI] [PubMed] [Google Scholar]
- 37. Liu Z, Habener JF. Glucagon-like peptide-1 activation of TCF7L2-dependent Wnt signaling enhances pancreatic β cell proliferation. J Biol Chem. 2008;283(13):8723–8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. da Silva Xavier G, Mondragon A, Sun G, et al. . Abnormal glucose tolerance and insulin secretion in pancreas-specific Tcf7l2-null mice. Diabetologia. 2012;55(10):2667–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Takamoto I, Kubota N, Nakaya K, et al. . TCF7L2 in mouse pancreatic β cells plays a crucial role in glucose homeostasis by regulating β cell mass. Diabetologia. 2014;57(3):542–553. [DOI] [PubMed] [Google Scholar]
- 40. Zhou Y, Park SY, Su J, et al. . TCF7L2 is a master regulator of insulin production and processing. Hum Mol Genet. 2014;23(24):6419–6431. [DOI] [PMC free article] [PubMed] [Google Scholar]