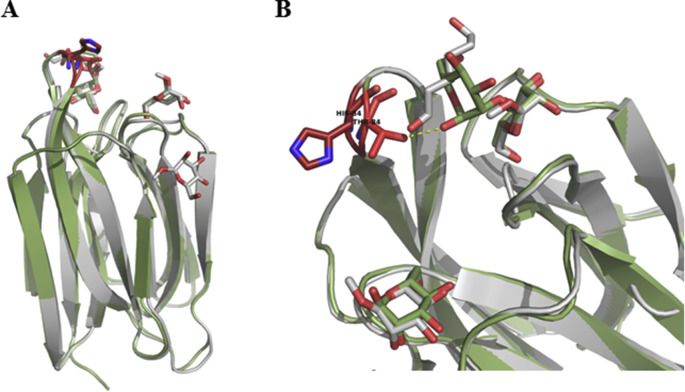
Fig. 5.
Superposition of BanLec WT and H84T Mutant Structures. A) The WT (grey) and H84T mutant (green) structures were aligned with a root mean squared deviation of 0.442 Å over 138 C-α positions. Mannose and dimannose are rendered as sticks. Residue 84 is rendered as sticks in the two structures and colored red. B) Close up of the loop containing residue 84 and BanLec CRD. Despite the decrease in mitogenicity, the overall fold of the protein is conserved with only the loop containing residue 84 repositioned with the C-α 1.4 Å closer and C-β 2.1 Å closer to the CRD in the mutant structure. This repositions results in an additional Hydrogen bond between 84T and dimannose hydroxyl.