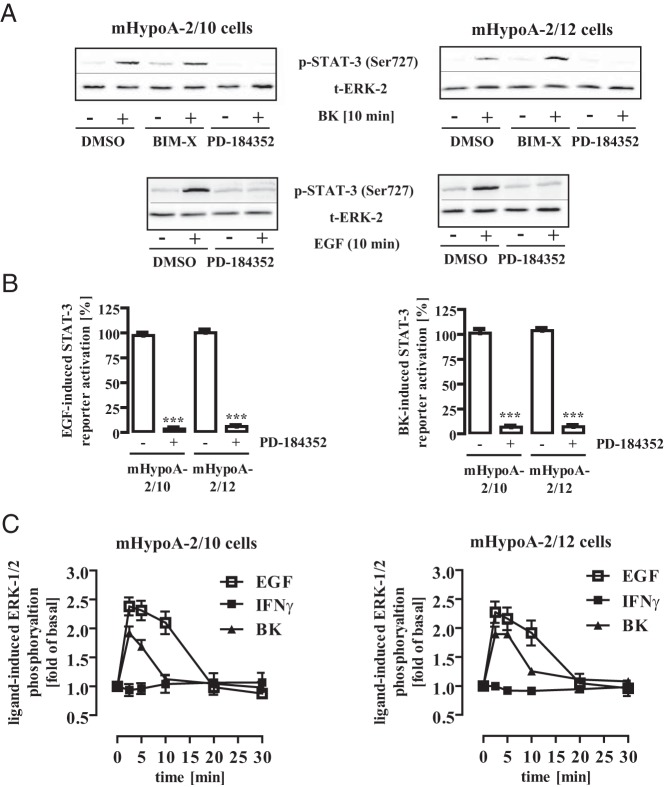
Figure 4. ERK-1/2 activity is required for BK- or EGF-induced STAT-3 activation.
A, mHypoA-2/10 or -2/12 cells were serum starved for 16 hours, preincubated for 30 minutes with BIM-X (1 μM), PD-184352 (10 μM), or the carrier DMSO (0.1%) and were then stimulated for 10 minutes with either BK (1 μM) or EGF (10 ng/mL). Cell lysates were subjected to Western blot analysis using either a phospho-specific antiserum against Ser-727 or against the total ERK-2 protein (t-ERK-2) to control for the total protein amount. One representative blot is shown. Data from 7 independent experiments are given in Table 1. B, mHypoA-2/10 or -2/12 cells were transfected with a STAT-3–dependent reporter gene construct, serum starved for 16 hours, preincubated for 30 minutes with PD-184352 (10 μM) or the carrier DMSO (0.1%), and then stimulated for 6 hours with either 10 ng/mL EGF (left panel) or 1 μM BK (right panel). The effects of PD-184352 on STAT-3 activation in 5 independent experiments performed in quadruplicate were compiled by defining ligand-induced reporter activation in the presence of DMSO as 100%. Ligand-induced STAT-3 activation is expressed as the mean ± SEM, and asterisks indicate a significant difference from 100%. C, mHypoA-2/10 (left panel) or mHypoA-2/12 (right panel) cells were serum starved for 16 hours and then were stimulated with IFN-γ (10 ng/mL), BK (1 μM), or EGF (10 ng/mL) for the indicated period of time. ERK-1/2 phosphorylation was detected by performance of a whole-cell ELISA with a p-ERK-1/2–specific antiserum. Ligand-induced ERK-1/2 phosphorylation measured in 5 independent experiments performed in quadruplicate was normalized to basal and is expressed as the mean ± SEM.