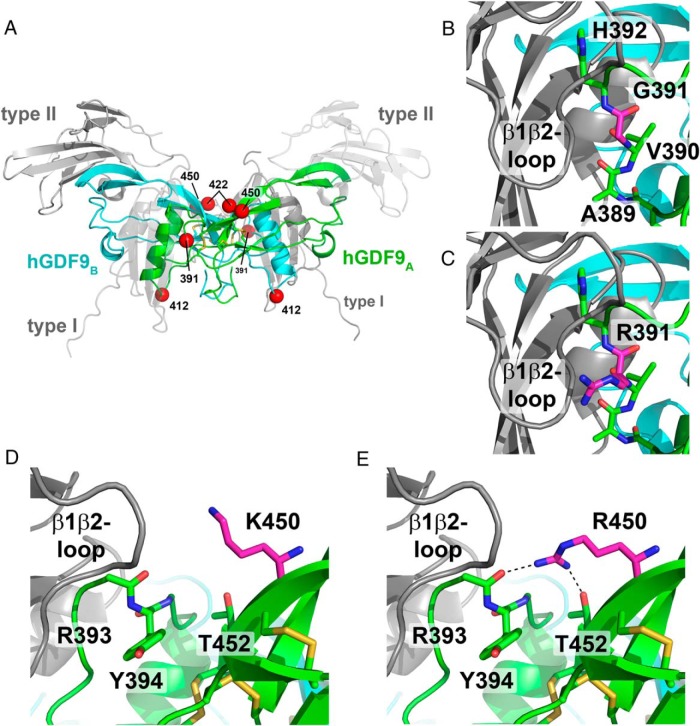
Figure 7. Homology model of the mature region of hGDF9 in complex with its type I and type II receptors.
A, Overview ribbon plot of the hGDF9 dimer with the monomeric subunits colored in green and cyan. The potential receptor binding sites are indicated by gray ribbon structures for the type I and type II receptor ectodomains; for better visibility, the type I and the type II receptors facing the front are shown as transparent structures. Disulfide bonds of the cystine knot are shown as yellow sticks; the position of the residues exchanged to form the human-mouse chimera are indicated by red spheres. B and C, The residue at position 391 is located within the potential type I receptor binding site. Docking of a type I receptor to hGDF9 suggests that the residue at 391 is near the receptor's β1β2-loop, which was shown to be important in ligand-type I receptor interactions. In human GDF9, a small glycine residue occupies position 391 (B), whereas in mouse GDF9 a large positively charged arginine requires more space (C) but is capable of forming additional hydrogen bonds with the type I receptor. D and E, A second mutation in the human-mouse GDF9 chimera M2 and M4 is the exchange of Lys450 to arginine. As for the former substitution, this exchange could also enable additional interactions due to the longer side chain and the bidentate polar head group.