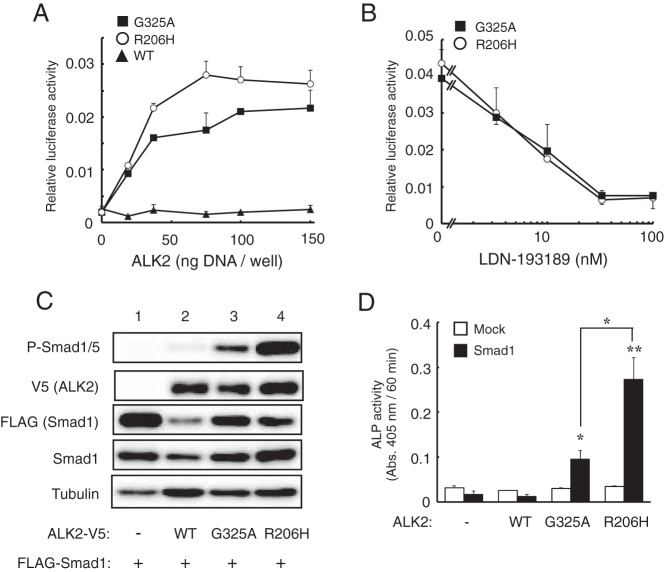
Figure 1. Overexpression of ALK2(G325A) in C2C12 cells induces BMP activity via the phosphorylation of Smad1/5.
A, BMP-specific luciferase reporter activity induced by mutant ALK2. C2C12 cells were cotransfected with increasing amounts of wild-type ALK2, ALK2(G325A) or ALK2(R206H) and a BMP-specific reporter plasmid, Id1WT4F-luc. After 24 hours, the activities of firefly and renilla luciferase were determined. The data are expressed as the mean ± SD (n = 3). B, Inhibition of mutant ALK2 activity by a kinase inhibitor of BMP receptors. C2C12 cells transfected with ALK2 and the Id1WT4F-luc reporter were treated overnight with or without increasing amounts of LDN-193189 (0–100 nM). The data are expressed as the mean ± SD (n = 3). C, Western blot analysis of phosphorylated Smad1/5. C2C12 cells were cotransfected with WT or mutant V5-ALK2 and FLAG-Smad1. Whole-cell lysates were analyzed with antibodies against phospho-Smad1/5, V5-tag, FLAG-tag, Smad1, and tubulin. D, ALP activity induced by the cooperative activity of ALK2 and Smad1. C2C12 cells were cotransfected with WT or mutant ALK2 and Smad1. After 3 days, ALP activity was determined by measuring the absorbance at 405 nm. The data are expressed as the mean ± SD (n = 3). *, P < .05 and **, P < .01 vs mock-transfected cells (ANOVA).