Figure 8.
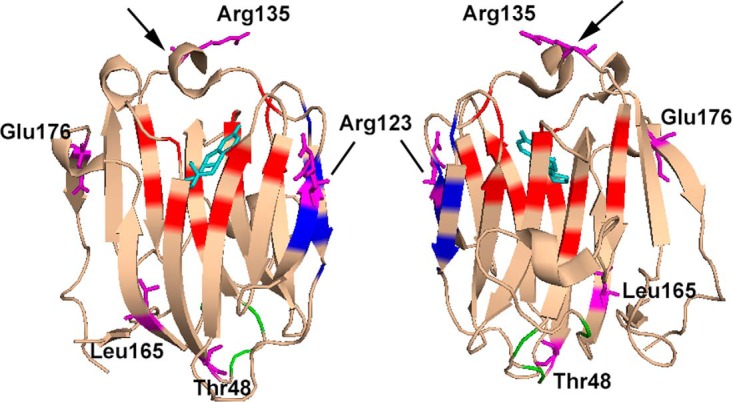
Crystal structure of the N-terminal LG domain of human SHBG in complex with estradiol (Protein Data Bank code: 1LHU; estradiol is shown in cyan) showing the residues where amino acid substitutions were found to alter the functional properties of SHBG. The positions of residues reported previously (5) to be involved in steroid binding (red), dimerization (blue), and calcium binding (green) are highlighted. The loop structure positioned above the steroid-binding pocket is indicted with an arrow. The residues where substitutions result in altered steroid binding (Thr48, Arg123, Arg135, Leu165, and Glu176), dimerization (Arg123 and Thr48), calcium binding (Thr48), or fibulin-2 binding (Thr48 and Arg135) are shown in purple. Note that Gly195 is not included in this crystal structure of the SHBG N-terminal LG domain.
