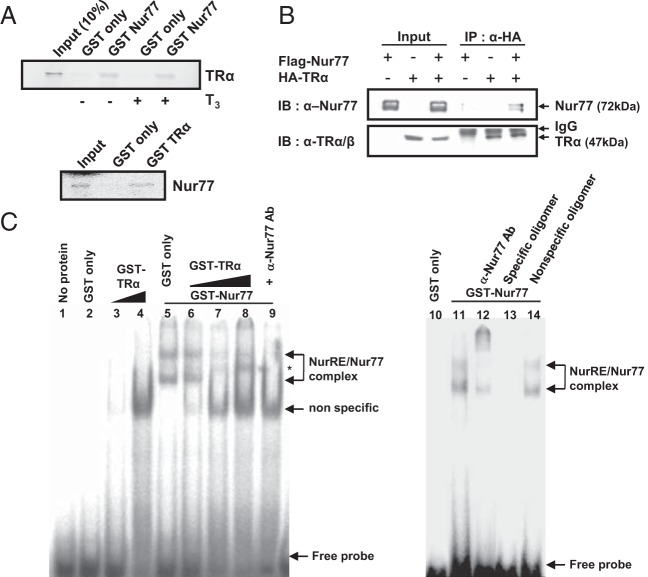
Figure 4.
Physical association of Nur77 with TRα. A, Nur77 directly interacts with TRα in vitro. [35S]methionine-labeled TRα and Nur77 produced by in vitro translation were allowed to interact with the GST-fusion protein of Nur77 and GST-fusion protein of TRα, respectively. B, Nur77 associates with TRα in cells. Cos-7 cells were cotransfected with flag-Nur77 and HA-TRα expression plasmids and coimmunoprecipitation was performed with anti-HA antibody. Western blot analyses were conducted with anti-Nur77 or anti-TRα/β antibody. C, The physical interaction of TRα with Nur77 on DNA was accessed by EMSA. The GST-Nur77 fusion protein was incubated with α-32P-labeled NurRE oligonucleotide, along with purified GST only (lane 5) or increasing amounts of GST-TRα (lanes 6, 7, and 8) proteins. A 50-fold excess of cold NurRE oligomer (lane 13) or nonspecific oligomer (lane 14) was added. Positions of the specific protein-DNA complex, nonspecific complex, and free probe were indicated. *, A new complex formed by coincubation with TRα protein. In all panels, data are representative of 3 independent experiments. IB, immunoblotting; IP, immunoprecipitation.