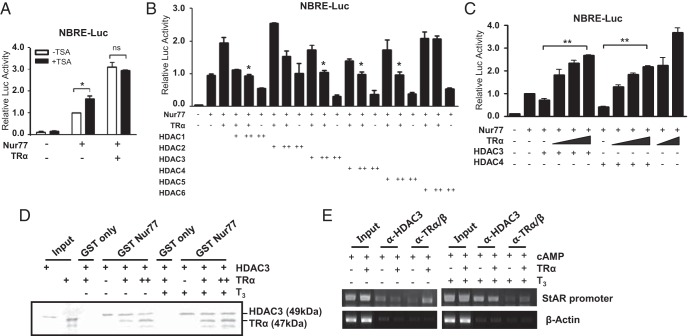
Figure 5.
Competition of TRα with HDACs for the modulation of Nur77 transactivation in the absence of T3. A, HDAC activity is involved in TRα-mediated enhancement of Nur77 transactivation in the absence of T3. MA-10 cells were transfected with Nur77 (50 ng) and TRα (25 ng) expression plasmids along with the NurRE-Luc reporter. After 24 hours of transfection, cells were treated with 100 nM of trichostatin A (TSA) and harvested for the measurement of luciferase activity after an additional 24 hours. B, TRα-mediated enhancement of Nur77 transactivation in the absence of T3 is inhibited by HDACs. Nur77 (50 ng), TRα (25 ng), and HDAC expression plasmids (+, 100 ng; ++, 200 ng) were transfected into MA-10 cells. Statistical significance compared with the control with Nur77 and TRα coexpression only. C, HDAC-suppression of Nur77 transactivation is relieved by coexpression. Nur77, increasing amounts of TRα (25, 50, and 100 ng; 25 and 100 ng), and the indicated HDAC expression plasmids (200 ng) were transfected into MA-10 cells. D, TRα competes with HDAC3 for binding to Nur77. The increasing amounts of in vitro-translated 35S-radiolabeled TRα were added to GST-Nur77 bound to glutathione-Sepharose beads and the constant amounts of in vitro-translated 35S-radiolabeled HDAC3. After washing, the bound proteins were eluted and separated by SDS-PAGE. E, TRα inhibits the recruitment of HDAC3 to the Nur77-binding site in the StAR promoter in the absence of T3. ChIP assays were performed using anti-HDAC3 and anti-TRα/β antibodies. In panels, A, B, and C, values are the means ± SEM of 3 independent experiments performed in duplicate. *, P < .05; **, P < .01; ns, not significant. In panels D and E, data are representative of 3 independent experiments.