ABSTRACT
Natural Killer (NK) cells are capable of recognizing and killing cancer cells and play an important role in tumor immunosurveillance. However, tumor-infiltrating NK cells are frequently impaired in their functional capability. A remarkable exception is represented by NK cells isolated from malignant pleural effusions (PE) that are not anergic and, upon IL2-induced activation, efficiently kill tumor cells. Although IL2 is used in various clinical trials, severe side effects may occur in treated patients. In this study, we investigated whether also other clinical-grade cytokines could induce strong cytotoxicity in NK cells isolated from pleural fluid of patients with primary or metastatic tumors of different origins. We show that PE-NK cells, cultured for short-time intervals with IL15, maintain the CD56bright phenotype, a high expression of the main activating receptors, produce cytokines and kill tumor cells in vitro similarly to those treated with IL2. Moreover, IL15-activated PE-NK cells could greatly reduce the growth of established tumors in mice. This in vivo antitumor effect correlated with the ability of IL15-activated PE-NK cells to traffic from periphery to the tumor site. Finally, we show that IL15 can counteract the inhibitory effect of the tumor pleural microenvironment. Our study suggests that IL15-activated NK cells isolated from pleural fluid (otherwise discarded after thoracentesis) may represent a suitable source of effector cells to be used in adoptive immunotherapy of cancer.
KEYWORDS: Adoptive immunotherapy, IL15, innate lymphoid cells (ILC), NK cells, pleural fluid, pleural tumors
Abbreviations
- ACT
adoptive cell therapy
- h
hours
- HD
healthy donors
- ILC
innate lymphoid cells
- i.v.
intravenously
- KIRs
killer immunoglobulin-like receptors
- mAb
monoclonal antibody
- NCR
natural cytotoxicity receptor
- NK
natural killer
- NSCLC
non-small cell lung cancer
- PB
peripheral blood
- PE
pleural effusions
- s.c.
subcutaneously
- spt
supernatants
- Treg
regulatory T cells
- un
unstimulated
Introduction
Natural killer (NK) cells belong to the family of innate lymphoid cells (ILCs).1,2 ILCs have been recently classified in two main groups according to their functional features and transcription factors required for their differentiation/survival: “cytotoxic-ILCs,” i.e., NK cells, and “helper-ILCs,” including ILC1, ILC2, and ILC3.3 NK cells are important effector cells of the innate immunity that play a relevant role in the defense against tumors and viruses. The antitumor activity of NK cells is based on their capability of recognizing and killing tumor cells, while sparing normal cells.4 The NK cell function is mediated by an array of activating receptors, including NKp46, NKp30, NKp44, NKG2D, and DNAM-1. These receptors recognize ligands that may be strongly upregulated or expressed de novo following tumor transformation. The function of activating receptors can be counteracted by signals delivered by killer immunoglobulin-like receptors (KIRs) or CD94/NKG2A inhibitory receptors upon interaction with HLA-class I molecules, expressed by normal cells but frequently downregulated by tumor cells.5,6,7,8,9,10,11,12,13,14,15,16
Two major NK cell subsets have been identified on the basis of the expression levels of CD56 surface molecules. CD56bright NK cells predominate in tissues, express CD94/NKG2A, and secrete various cytokines while they are poorly cytolytic. In contrast, CD56dim cells represent the majority of peripheral blood (PB) NK cells, may express KIRs, are highly cytolytic and can rapidly secrete cytokines upon crosslinking of their activating receptors.17,18,19
To design new therapeutic strategies based on adoptive cell therapy (ACT), many studies analyzed the functional proprieties and the correlation with prognosis of NK cells present in hematological and solid tumors.20,21,22,23 In particular, it has been shown that NK cells play a role in limiting the spreading of metastatic cells. Moreover, in solid tumors, high numbers of peripheral or tumor-associated NK cells correlate with a better prognosis.24-26,27,28,29,30 However, different reports revealed, in non-small cell lung cancers (NSCLCs), renal carcinomas and breast cancers, the presence of tumor-associated CD56bright NK cells that displayed a poor cytolytic activity against tumor cells.25,27,31-33
In a previous study, we analyzed NK cells present in pleural effusions (PE) of primary and metastatic tumors of various origins including mesothelioma and lung, breast, colon, gastric, bladder, and uterus carcinomas. In spite of their CD56bright phenotype, PE-NK cells expressed high levels of CD107a upon interaction with tumor cells, revealing a high cytolytic potential and indicating that PE-NK cells are not substantially inhibited by the tumor pleural microenvironment. In addition, upon IL2-induced activation, PE-NK cells lysed tumor cells more efficiently than IL2-activated autologous PB NK cells. These data suggested a possible use of IL2-activated NK cells to treat primary or metastatic pleural tumors by infusing cells systemically or in the pleural cavity.34,35,36 Moreover, the presence of NK cells in PE could also suggest a possible benefit by the infusion of IL2-alone directly in the pleural cavity. In support of this possibility, the results of a previous study showed that intrapleural IL2 administration, in a patient with PE associated with lymphoma, resulted in a long-lasting (over 2 years) absence of PE.37 On the other hand, several clinical studies based on the systemic infusion of high doses of IL2, showed limited clinical benefits.38,39,40 This is in part related to the fact that IL2 may not only induce proliferation and functional activation of tumor-specific effector cells, but also of regulatory T cells (Treg) leading to the impairment of the therapeutic effect.41,42 In addition, IL2 may induce a severe toxic effect resulting in the vascular leak syndrome.43 Thus, alternative cytokines, capable of effectively boosting NK cells without inducing suppressive loops (such as IL15, IL12, and IL18) are currently being investigated in preclinical cancer models.44,45,46 IL15 is a potent immunostimulating cytokine, potentiating both T and NK cell-mediated immune responses and promoting the generation of memory T cells.47,48,49,50 In particular, recent clinical trials identified IL15 as an alternative cytokine (replacing IL2) in the treatment of metastatic melanoma and renal carcinoma.51 Moreover, although different studies suggested that IL12 and IL18, when used alone, display a limited anticancer activity, the combined treatment with IL12 and IL18, revealed that the anergic state of tumor-infiltrating NK cells can be reverted.52,53,54 Taken together, these data suggest that the activity of endogenous NK cells can be enhanced in vivo by the systemic or local administration of cytokines, such as IL2, IL15, and IL12 plus IL18. Other strategies include the use of drugs capable of upregulating the activating NK receptors or their ligands on tumor cells, as well as the use of blocking antibodies directed to inhibitory NK receptors.39,55-57 These treatments may be combined with the infusion of clinical-grade cytokines.58 In addition, recent data suggested that adoptive immunotherapy, based on the administration of cytokines together with in vitro activated effector cells, may result in improved antitumor efficacy.52,59-61
Here, we analyzed the capability of different clinical-grade cytokines, besides IL2, such as IL15, IL12, and IL18, to potentiate the antitumor activity of PE-NK cells. We show that short-term IL15-activated PE-NK cells maintain the CD56bright phenotype, and the potential of migrating to tumor site. In addition, they display strong cytolytic activity, and cytokine production and efficiently control tumor growth in a murine model. Finally, we show that IL15 counteracts the inhibitory effect mediated by soluble factors present in malignant pleural fluid. These results may suggest new therapeutic strategies of adoptive immunotherapy in patients with PE associated with primary or metastatic tumors.
Results
Effect of different cytokines on the phenotypic and functional features of PE-NK cells
Our study is focalized on NK cells isolated from malignant PE (Table 1). The main aim is to establish culture conditions allowing the generation of cells, with potent antitumor activity, suitable for adoptive immunotherapy.
Table 1.
| Features of the 43 patients included in our study | n° |
|---|---|
| Male | 33 |
| median age 74 (range 55-91) | |
| Female | 10 |
| median age 74 (range 53-83) | |
| Mesothelioma | 32 |
| Adenocarcinoma Lung | 7 |
| Adenocarcinoma Gastrointestinal (colon and pancreas) | 4 |
In a first set of experiments, we evaluated the cell types present in PE, in terms of percentages of CD45+ cells, T, B, NK, and myeloid cells (Fig. S1). Notably, NK cells represented 9.48 ± 4% of CD45+ cells.
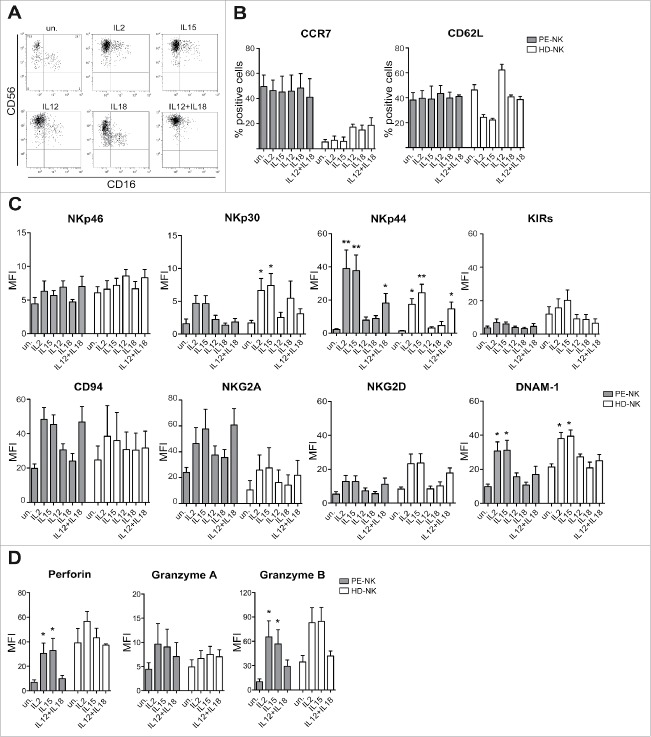
Next, we analyzed the effect of different clinical-grade cytokines currently used in preclinical cancer models, on the phenotypic properties and on the effector function of PE-NK cells. To this end, PE-NK cells or NK cells isolated from PB of healthy donors (HD-NK), used as controls, were cultured for 72 h with IL2, IL15, IL12, and IL18, used alone or in combination. As shown in Fig. 1A, PE-NK cells retained the CD56bright phenotype and did not acquire CD16 upon culture with all the cytokines analyzed. In addition, upon cytokine stimulation, they maintained the expression of CD62L and CCR7, two receptors involved in cell migration (Fig. 1B). On the contrary, in HD-NK cells, the expression of CD62L decreased upon culture with IL2 or IL15, while it increased upon culture with IL12 (in agreement with a previous report62). In HD-NK cells (Fig. 1B), the expression of CCR7 was confined to CD56 bright cells (not shown). In addition, PE-NK cells, cultured with either IL2 or IL15, expressed higher levels of NKp30, NKp44, and DNAM-1 activating receptors than unstimulated (un) or IL12- or IL18-cultured PE-NK cells. In particular, the combination of IL12 and IL18 induced only a partial upregulation of NKp44, NKG2D, and DNAM-1, while the expression of NKp30 was unchanged (Fig. 1C). The expression of NKp46, NKG2D, CD94, NKG2A, and KIRs were not significantly modified upon culture with all the cytokines analyzed as compared with unstimulated cells. Similar results were obtained in parallel cultures of HD-NK cells (Fig. 1C). Analysis of cytolytic granules indicated that IL2- or IL15-cultured PE-NK cells displayed higher levels of perforin and granzymes A and B than those cultured with IL12+IL18 or unstimulated (Fig. 1D).
Figure 1.
Cytokine-stimulation induces phenotypic changes in PE-NK cells. (A–D) Freshly isolated PE- (gray bars) and peripheral blood HD-NK (white bars) cells were stimulated with IL2, IL15, IL12, and IL18 alone or in combination, for 72 h (short-term) and then analyzed. (A) CD56 and CD16 surface expression on unstimulated (un.) and on cytokine-stimulated PE-NK cells. One representative experiment out of 12 performed. (B) Percentages ± SEM of CCR7 and CD62L expression (n = 4). (C) Mean fluorescence intensity (MFI) ± SEM of the major activating and inhibitory NK receptors (n = 8). (D) MFI ± SEM of perforin, granzymes A, and B (n = 4). (B–D) Statistical analysis was performed using one-way ANOVA Kruskal–Wallis test, (*) indicates the comparison between cytokine stimulated and unstimulated NK cells within the same group (PE-NK or HD-NK). Where not indicated, data were not statistically significant.
We next analyzed the effect of these cytokines on PE-NK cell function. As target cells, we used A549 adenocarcinoma tumor cell line that expresses the ligands recognized by major activating NK receptors (Fig. 2A). In particular, A549 tumor cells were characterized by high levels of expression of PVR, Nectin-2 (DNAM-1 ligands), and intermediate/low levels of ULBP1–3, MICA, and MICB (NKG2D ligands). The B7H6 molecule (NKp30 ligand) was not detected. In functional experiments, PE-NK cells were cultured for 72 h in the presence of IL2, IL15, IL12, and IL18, used alone or in combination and their ability to undergo degranulation (CD107a positivity) and to produce cytokines (IFNγ and TNF) were analyzed upon NK cell exposure to adenocarcinoma A549 tumor cells. As shown in Figs. 2B–D, IL2- or IL15- or IL12+IL18-activated PE-NK cells displayed a higher CD107a surface expression and cytokine production than unstimulated or IL12- or IL18-cultured PE-NK cells. Similar results were obtained using HD-NK cells, as effector cells, and K562 as tumor target cells (Figs. 2B–D).
Figure 2.
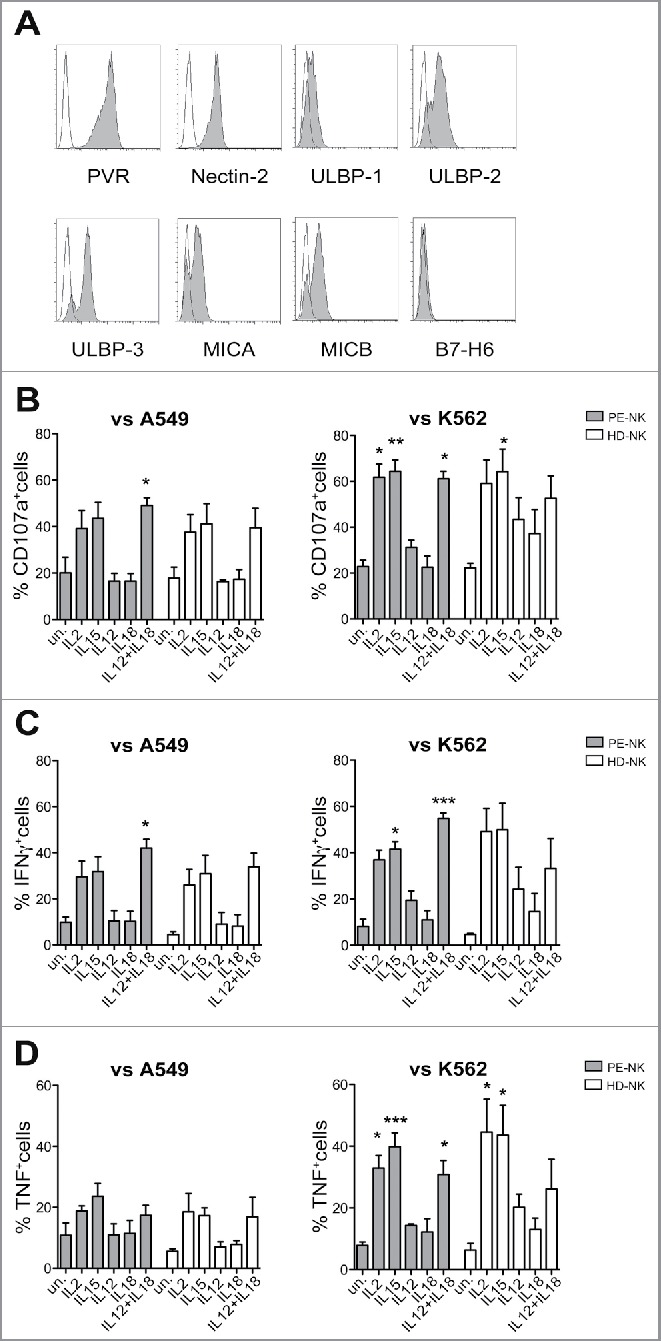
Cytokine stimulation upregulates PE-NK cell function. (A) Surface expression of cell ligands recognized by activating NK receptors on A549 tumor cell line. One representative experiment out of four performed. (B–D) NK cells isolated from PE (gray bars) or from peripheral blood of HD (white bars) were stimulated for 72 h (short-term) with IL2, IL15, IL12, and IL18, alone or in combination. (B) Percentages ± SEM of CD107+ NK cells upon exposure to A549 (n = 5) or K562 target cells (n = 6). (C, D) Percentages ± SEM of IFNγ+ or TNF+ NK cells upon exposure to A549 (n = 5) or K562 target cells (n = 6). (B–D) Statistical analysis was performed using one-way ANOVA Kruskal–Wallis test, (*) indicates the comparison between cytokine-stimulated and unstimulated cells within the same group (PE-NK or HD-NK). When not indicated, data were not statistically significant.
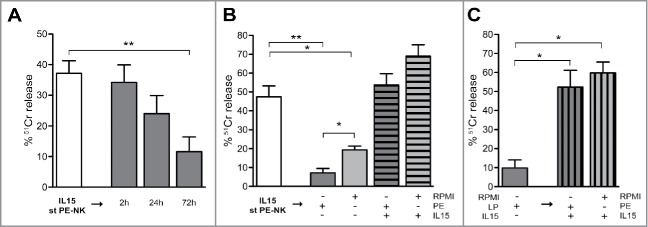
We also assessed the cytolytic activity of PE-NK cells using a 51Cr release assay. IL15-cultured PE-NK cells efficiently killed A549 target cells (Fig. 3A), while cells cultured with IL2 or IL12+IL18 displayed a lower cytolytic activity. The use of K562 as target cells gave similar results (Fig. 3B). Thus, our data indicate that IL15, similarly to IL2, induces a substantial upregulation of PE-NK cell effector function, whereas a combination of IL12+IL18, although it induces an increase of cytokine production, was not able to potentiate a suitable cytolytic activity of PE-NK cells.
Figure 3.
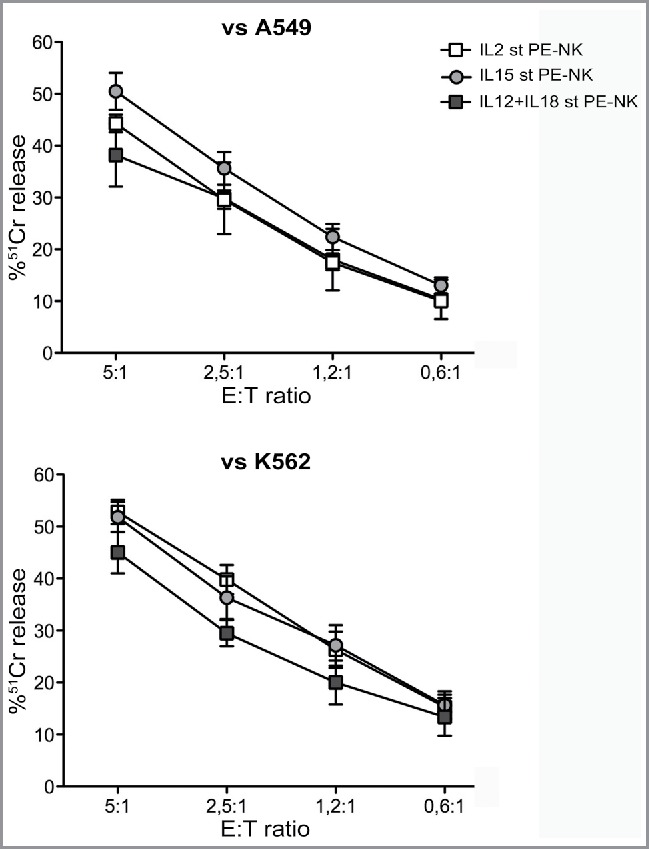
Short-term IL15-activated PE-NK cells display efficient cytolytic activity. Cytolytic activity of IL2, or IL15 or IL12+IL18 short-term (st) PE-NK cells was evaluated. Mean of 51Cr release ± SEM using A549 (n = 6–8) or K562 (n = 5–6) as target cells. The Effector:Target (E:T) ratios are indicated.
IL15-activated PE-NK cells control tumor growth in vivo
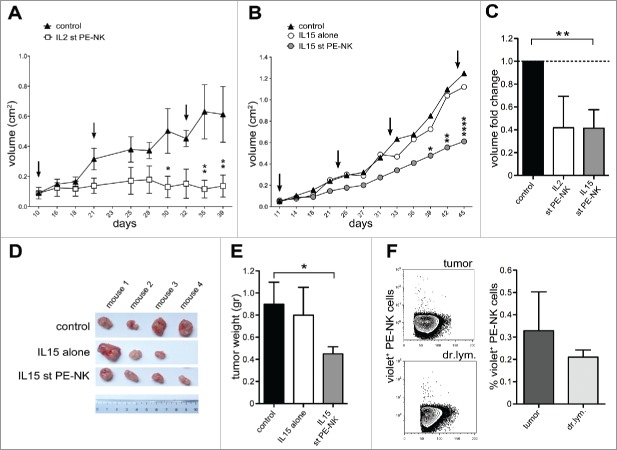
Based on the results above indicating that IL15, similar to IL2, can potentiate the cytolytic activity of short-term PE-NK cells in vitro, we further investigated whether IL15-activated cells could mediate antitumor activity in vivo. In these experiments, A549 tumor cells were injected subcutaneously (s.c.) in athymic nude mice and tumor growth was assessed every 2 d. Palpable tumors were detected 7–9 d after inoculation. Cytokine-activated PE-NK cells were infused intravenously (i.v.) at 10-d intervals starting at day 10 (IL2) or 11 (IL15), after tumor inoculation. In mice treated with either short-term IL2- or IL15-PE-NK cells tumor growth was efficiently controlled (Figs. 4A–C). In mice that did not receive NK cells, tumors further expanded (Figs. 4A–B). Notably, the ability of IL15-activated PE-NK cells to inhibit tumor growth in vivo was comparable, or better, to that of IL2-activated PE-NK cells (Fig. 4C).
Figure 4.
IL15-activated PE-NK cells controlled tumor growth in vivo. (A–F) A549 were injected subcutaneously (s.c.) into athymic nude mice (day 0). At different time intervals mice were treated with PBS (control), or IL15 alone (IL15), or with PE-NK cells pre-activated by IL2- or IL15-short-term (st) culture (72h). (A) Mice were treated every 11 d (see arrows) with IL2 st PE-NK cells (white symbols) derived from same patient or with PBS (black symbols), starting when tumors were palpable (day 10). Data indicate the mean tumor volume ± SEM (n = 4 per group).(B) Mice were treated every 11 d (see arrows) with IL15 st PE-NK cells (gray symbols, n = 4) derived from same patient, or with IL15 alone (white symbols, n = 3), or with PBS (black symbols, n = 4), starting when tumors were palpable (day 11). Data indicate the mean tumor volume. (A, B) Statistical analysis was performed using two-way ANOVA test, (*) indicates the comparison between mice infused with IL2- or IL15-short-term PE-NK cells and the corresponding PBS-treated mice group .Where not indicated, data are not statistically significant. (C) Fold change ± SEM of tumor volume (at day 39) of IL2- or IL15-short-term PE-NK cells treated group calculated on the basis of the tumor volume detected in control group (PBS), arbitrarily normalized to 1. Statistical analysis was performed using Mann–Whitney test, comparing treated mice with control group. Where not indicated, data are not statistically significant.(D) Representative image of tumors isolated from mice at day 45, each treatment group is indicated. (E) Mean tumor weight ± SEM was measured at day 45 in groups of mice treated as indicated. Statistical analysis was performed using Mann–Whitney test, comparing mice treated with IL15-activated PE-NK cells or IL15 alone with control group. When not indicated, data were not statistically significant. (F) Recruitment of IL15 st PE-NK cells in tumors and tumor draining lymph nodes (dr.lym.) in the corresponding treated mice. One representative experiment and percentages ± SEM of violet+ NK cells recovered (n = 4).
Athymic nude mice, while lacking mature T cells, still have functional NK cells.63 Thus, to exclude the possible contribution of autologous murine NK cells to the control of tumor growth, we analyzed whether they were present at the tumor sites, in tumor-draining lymph nodes and spleen (Figs. S2A and B). Mice treated under different conditions showed similar percentages of autologous NK cells in the various tissues analyzed. We also evaluated their stage of maturation in terms of expression of CD27 and CD11b, two markers that allow to identify four developmentally related murine NK cell subsets. As shown in the Figs. S2A and C, the maturation level of murine NK cells present at the tumor sites, tumor-draining lymph nodes, and spleen was comparable among the different groups of treated mice. These data, together with the results of control mice that did not receive cytokine activated PE-NK cells (Figs. 4A–E), strongly suggest that autologous murine NK cells do not influence tumor growth and their numbers and phenotypic features were not affected by treatment with cytokine-activated PE-NK cells or with IL15 alone (used as additional control). Therefore, it is conceivable that only infused cytokine-activated PE-NK cells mediate the control of tumor growth.
Since IL15, different from IL2, does not induce toxicity, we focused our investigation on IL15-activated PE-NK cells, using IL15 alone as control. In these experiments, both tumor size and weight were significantly lower in IL15 PE-NK cell-treated mice than in mice either untreated or treated with IL15 alone (Figs. 4B, D, and E). One of the mice treated with IL15 alone was not included in the results because it was killed before the end of the experiment due to an excessive tumor growth. These data suggest that PE-NK cells that have been activated in vitro with IL15 can control tumor growth in vivo.
We next analyzed whether the antitumor effect of IL15-activated PE-NK cells correlated with their ability to migrate to the tumor site. To this end, these cells were labeled with violet dye and their tissue distribution was analyzed one day after injection in mice at the end of the in vivo experiments. As shown in Fig. 4F, violet+ NK cells were present both at the tumor site and in the tumor-draining lymph nodes. These data indicate that infused PE-NK cells can efficiently traffic from the periphery to the tumor site where they may exert their effector function.
IL15 is required to maintain the antitumor activity of PE-NK cells
It is well known that the tumor microenvironment may contain different soluble immunomodulatory factors responsible of the impairment of NK cell function.64,65,66 Thus, we analyzed if cytokines, chemokines, or soluble ligands of NK receptors were present in PE, as well as in supernatants (spt) of autologous tumor cell line culture. As shown in Fig. 5, PE-tumor cells can release the same soluble factors detectable in the pleural fluid. Thereafter, we investigated whether malignant PE could affect the cytolytic activity of NK cells. To this end, we compared the cytolytic activity of IL2- or IL15-activated HD-NK cells in the absence or in the presence of PE. HD-NK cells that had been cultured with cytokines alone, displayed a sharp cytolytic activity that was not affected in the presence of PE (Fig. S3). On the basis of these results, we focused our investigation on IL15-activated NK cells. Thus, we further assessed whether autologous PE could inhibit the cytolytic activity of PE-NK cells that had been pre-activated with IL15 (for 72 h). To this end, IL15 short-term PE-NK cells were cultured with autologous PE for different time intervals (2 h, 24 h, 72 h) and their ability to kill A549 tumor cells was assessed. Incubation for 2 h did not affect the cytolytic activity (Fig. 6A). However, after 24 h incubation, the cytolytic activity of IL15-pre-activated PE-NK cells was substantially decreased, while after 72 h it was virtually abrogated (Fig. 6A). To verify whether the impairment of the PE-NK cytolytic potential was exclusively due to the effect of soluble factors present in PE or rather reflect the absence of IL15 from cultures, IL15-pre-activated PE-NK cells were cultured also in medium alone. After 72 h, the NK cytotoxicity was partially compromised, however, the functional impairment was more marked in the presence of PE (Fig. 6B). Notably, when pre-activated PE-NK cells were cultured for additional 72 h with both IL15 and PE, their cytolytic activity was not affected (Fig. 6B). These data suggest that soluble components present in PE may compromise the functional capabilities of pre-activated PE-NK cells; however, IL15 can efficiently counteract the inhibitory effect of PE. We also investigated whether IL15 was able to restore the cytolytic activity of pre-activated PE-NK cells that had been inhibited by culture with PE for 72 h. As shown in Fig. 6C, cytolytic activity was recovered by the addition of IL15 both in the presence and in the absence of PE.
Figure 5.
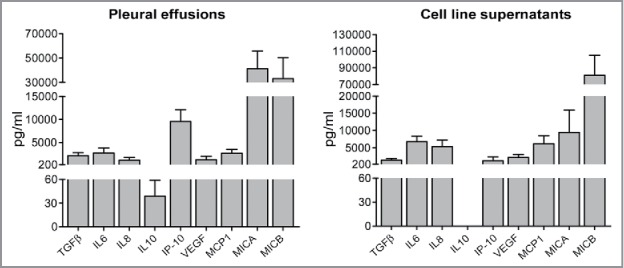
Soluble factors detected in malignant pleural effusions and in autologous tumor cell line supernatants. Malignant pleural effusion and their autologous tumor cell line culture supernatants were analyzed by ELISA and Luminex multiplex assays. Mean ± SEM of cytokine concentration (pg/mL) (n = 5).
Figure 6.
IL15 counteracts the inhibitory effect of malignant pleural fluid. (A, B) IL15 short-term (st) PE-NK cells were cultured under different culture conditions and their cytolytic activity against A549 cells was analyzed (E:T ratio, 5:1). Pre-activated IL15 st PE-NK cells (white bar) were washed and subsequently: (A) cultured with autologous PE for different time intervals (2 h, 24 h, and 72 h) (gray bars) (n = 5); (B) cultured for 72 h with the following: autologous PE (dark gray bar), RPMI (light gray bar), autologous PE+IL15 (dark gray stripped bar), and RPMI+IL15 (light gray stripped bar) (n = 3–7). (C) Pre-activated IL15 st PE-NK cells cultured subsequently for 72 h with autologous PE (dark gray bar) were washed and cultured for additionally 72 h with IL15 and autologous PE or RPMI (dark and light gray stripped bars, respectively) (n = 3). (A–C) Percentages ± SEM of 51Cr release. Statistical analysis was performed using Mann–Whitney test. Where not indicated data were not statistically significant.
Taken together, these data suggest that IL15, in addition to its ability to overcome the inhibitory effect of soluble factors present in pleural fluid, may also restore the effector function of PE-NK cells that has been compromised by the exposure to autologous malignant pleural fluid.
Discussion
Our present study shows that NK cells isolated from malignant pleural fluid (PE) obtained from patients with primary or metastatic tumors of different origin, acquire strong effector function upon short-term activation in vitro with IL2 or IL15. Moreover, the ability of IL15-activated PE-NK cells to control tumor growth in vivo suggests that they may represent a suitable source of potent antitumor effector cells to be used in adoptive immunotherapy of tumors. Since the inhibitory effect exerted by malignant pleural fluid on PE-NK cell function could be restored by IL15, the use of this cytokine for the treatment of patients with primary or metastatic intrapleural tumors should be carefully considered.
he characteristics of lymphoid cell infiltrates in tumor tissues may have a predictive diagnostic and prognostic value.24,65,67,68 In particular, a better clinical outcome correlates with an infiltrate rich of cytotoxic cells (NK and T cells).25,26,50,69 Notably, malignant pleural fluids, obtained from thoracentesis in patients with mesothelioma or other tumors with pleural localization, contain relatively high numbers of lymphoid cells, including NK cells.34,36 Since thoracentesis is a necessary therapeutic maneuver in many patients with a pleural involvement, the pleural fluid, otherwise discarded, may be used as a source of autologous NK cells for adoptive immunotherapy. As previously shown, PE-NK cells were not anergic and, upon IL2 activation, displayed a cytolytic activity against tumor cells even higher than that of autologous IL2-activated PB-NK cells.34 It is well established, in a large number of clinical trials, that IL2 has relevant toxic effects and induces Treg expansion that may compromise the antitumor responses.21,38-41,43 Therefore, we investigated whether also other clinical-grade cytokines, known to induce NK-cell activation, including IL15, IL12, and IL18, could trigger the PE-NK cell effector function. Our present data indicate that, upon cytokine stimulation, PE-NK cells maintain their CD56bright CD16− phenotype and increase their effector function. In particular, IL12 and IL18 could induce PE-NK cell activation only when used in combination, but their activity was mainly confined to cytokine production. Instead, IL15, similarly to IL2, could enhance the cytolytic activity of PE-NK cells in vitro. Based on these in vitro data, we further investigated the antitumor effect of IL15- or IL2-stimulated PE-NK cells in a murine model. We show that IL15 short-term PE-NK cells display a similar if not better ability to control tumor growth in vivo as compared with those activated with IL2. In light of these results and of the low toxic effect of IL15, we focused our studies on IL15-activated PE-NK cells. Thus, we attempted to establish culture conditions for generating NK cells suitable for effective applications in adoptive immunotherapy. It is known that a prolonged in vitro exposure of NK cells to cytokines allows expansion and recovery of large number of cells but may induce NK cell exhaustion and compromise their functional activities. Therefore, we investigated the effect of short-term NK cell activation with different cytokines. We show that after 72 h of culture with IL15, PE-NK cells, while not increasing in number, acquire a strong cytolytic activity and a good capability of cytokine production. This approach also minimizes cell manipulations and the risk of culture contaminations.
Previous studies in patients with solid tumors have reported the presence of low NK cell numbers in tumor infiltrates, suggesting an impaired ability of these cells to migrate to the tumor site.67,70,71 Since short-term IL15 PE-NK cells maintain not only the CD56bright phenotype but also the expression of surface receptors (CCR7 and CD62L) involved in cell migration to tissues, they may be recruited to tumor sites. This is also supported by our present data in a murine model. Accordingly, it is possible to envisage the use of IL15-stimulated PE-NK cells in systemic adoptive immunotherapy. However, a loco-regional cell infusion may allow to reach higher cell concentrations and to use lower numbers of NK cells that can exert an effective antitumor activity directly in the pleural cavity.
It has been shown that the tumor microenvironment contains many soluble and cell-bound factors capable of modulating the antitumor activity of NK cells.64 Although such immunomodulatory effect has been described both in solid and in hematologic tumors, limited information existed on the NK cells present in malignant PEs. In this study, we show that tumor PE contain inhibitory factors and that NK cells, exposed to autologous PE, may be impaired in their cytolytic activity. However, IL15 could counteract the inhibitory effect of the tumor pleural microenvironment and restore the functional capability (i.e., cytolytic activity) of NK cells. Based on these results and since IL15 does not cause relevant toxicity and does not induce Treg expansion, only this cytokine was deeply analyzed in our study. Notably, our data indicate that IL15, not only overcomes the inhibitory effect of soluble factors present in PE, but can also restore the functional activity of PE-NK cells that had been compromised by the exposure to PE.
In conclusion, our present study may help to design new approaches to treat cancer patients based on NK-cell-based ACT and may offer an interesting clue for the clinical use of PE-NK cells. Moreover, we highlight the high efficacy of IL15 that is capable not only of inducing optimal activation of NK cells to be used in adoptive immunotherapy, but also of preventing the inhibitory effect exerted by pleural fluid and, possibly, by the microenvironment of different tumors.
Materials and methods
Patients
All patients (Table 1) included in the study showed PEs associated with by primary or metastatic pleural tumors. Diagnosis was confirmed by demonstration of malignant cells in pleural fluid (cytopathological test) or by biopsy. We collected 43 PE obtained by thoracentesis technique. PE cells were obtained by centrifugation at 400 g for 10 min and preserved in 10% serum-supplemented RPMI 1640 medium (BioWhittaker, Lonza). This study was approved by Azienda Sanitaria Locale 3 (ASL, Genova, Italy) Ethics Board (ID 33533184, 29/10/2013). PB of healthy donors (HD) from buffy coat (UO Centro Trasfusionale, IRCCS AOU San Martino-IST) was used as controls. All patients gave written informed consent in according to the Declaration of Helsinki. In each group of experiments were used different samples, maintaining the proportion/ratio of mesothelioma vs. adenocarcinoma in accordance to patients involved in this study (Table 1).
Isolation and culture of NK cells
Lymphocytes from PE and from PB of HD were obtained by density gradient Ficoll-Hypaque (Lympholyte-H, Cederlane). NK cells were isolated using the NK cell isolation kit II (Miltenyi Biotec). We worked according to data sheets and cell purity was analyzed by flow cytometry: the population displayed more than 95% of CD56+CD3− phenotype. Short-term cells were obtained culturing PE- or HD-NK cells for 72 h in 10% serum-supplemented RPMI 1640 medium with IL2 (Proleukin Chiron, final concentration 600 UI/mL), IL15 (Miltenyi Biotec, final concentration 10 ng/mL), IL12 (Miltenyi Biotec, final concentration 0.1 ng/mL), and/or IL18 (MBL International Corporation, final concentration 0.1 μg/mL), used alone or in combination.
Cell lines
Human A549 lung adenocarcinoma cells were provided from CRB-IST cell bank ICLC (www.iclc.it) and authenticated by PCR/STR (Short Tandem Repeat) analysis. Human K562 erythroleukemia cells were authenticated by CRB-IST cell bank ICLC with an STR profile. Both cell lines were used within 6 mo of resuscitation of original cultures. Tumor cell lines from patients with malignant PE were obtained as described previously34
Immunofluorescence and antibodies
To perform immunofluorescence, human or mouse cells were stained with specific mAb for 30 min at 4°C. Before staining with mAbs, murine cells were incubated with FcR-blocking reagent (Miltenyi). We used the following directly conjugated mAbs: CD3 PE/Dazzle, anti-mouse CD3 Dazzle594, anti-mouse CD27 PE, CD226 PE (anti-DNAM-1), and CD94 FITC were purchased from BioLegend; CD16 FITC, CD314 PE (anti-NKG2D), CD107a APC, CD14 PE, and CD45 APC Vio770 were purchased from Miltenyi Biotec; CD62L FITC was purchased from BD Bioscience; CD3 ECD, CD56 Pc7, CD159a PE (anti-NKG2A), CD335 PE (anti-NKp46), CD336 PE (anti-NKp44), CD337 PE (anti-NKp30), CD158a,h PE (anti-KIRDL1-S1), CD158b1/b2,j PE (anti-KIR2DL2/3-S2), CD158e1/e2 PE (anti- KIR3DL1-S1) were purchased from Beckman Coulter. In the analysis of inhibitory receptors (Fig. 1C) KIRDL1-S1, KIR2DL2/3-S2, KIR3DL1-S1 were indicated as KIRs; CD19 alexaFluor450, anti-mouse NKp46 eFluor660, and anti-mouse CD11b PE-Cy7 was purchased from eBioscience. For indirect immunofluorescence, cells were incubated with specific mAb and then with isotype-specific goat anti-mouse second reagent (PE conjugated, Southern Biotechnology Associates). We used these specific mAbs: 170818 (IgG2a, anti-ULBP1), 165903 (IgG2a, anti-ULBP2), 166510 (IgG2a, anti-ULBP3), 150503 (IgG2a, anti-CCR7) purchased from R&D System; BM02 (IgG2a, anti-MICB) purchased from BamOmAb; L14 (IgG2a, anti-Nectin-2), L95 (IgG1, anti-PVR), and BAM195 (IgG1, anti-MICA) kindly provided by D. Pende (Genova, Italy); anti-B7H6 (IgG1) kindly provided by E. Vivier. For intracytoplasmic staining, cells were fixed and permeabilized with Cytofix/Cytoperm kit (BD Biosciences) and stained with directly conjugated mAbs: IFNγ PE and granzyme A PE (BD biosciences), TNF Brilliant Violet (eBioscience), perforin PE (Ancell), granzyme B PE (Invitrogen). In all samples, LIVE/DEAD Fixable Aqua Dead Cell Stain Kit 405 nm excitation (Molecular Probes) was added. Unstained cells were used as negative controls. Samples were analyzed on a Gallios Flow Cytometer (Beckman Coulter) and data analysis was performed using FlowJo 8.8.6 software (TreeStar Inc.).
Functional features
CD107a expression (degranulation activity) and the production of IFNγ and TNF were analyzed on short-term PE- and HD-NK cells upon interaction with A549 or K562 tumor cell lines. NK cells were co-cultured for 4 h with target cells (Effector:Target (E:T), 1:1) in the presence of CD107a mAb and monensin-containing GolgiStop (BD Biosciences, final concentration of 2 mM). Therefore, cells were harvested, washed, and stained with specific surface/intracellular mAbs. To perform cytotoxicity assays, short-term cultured PE-NK cells were tested for cytolytic activity using 51Cr-release assay against A549 and K562 tumor cell lines at different E:T ratios.
Mice experiments
NOD-SCID mice were purchased from Harlan (Udine, Italy). Mice were maintained at the Animal Facility of the IRCCS-AOU San Martino-IST. All mice were used between 6 and 12 weeks of age. Housing and treatments of animals were in accordance with the Italian and European Community guidelines (D.L. 2711/92 No.116; 86/609/EEC Directive) and approved by the internal Ethic Committee (ID 238/2015-PR, Ministero della Salute). Mice were killed by cervical dislocation. A549 tumor cells were injected subcutaneously (s.c.) at 10 × 106 cells/mouse and the tumor growth was monitored every 2 d. When tumors were palpable we started the treatments with short-term IL2- or IL15-activated PE-NK cells. Short-term PE-NK cells derived from same patient were infused intravenously (i.v.), 7 × 105 cells/mouse, at 10/11 d intervals. IL15 alone was administered by intraperitoneal injection at 10 ng/mice. For each set of experiments, mice were equally divided in subgroups with same number of mice and size tumor. All experiments included PBS-injected control mice. In one set of experiment, short-term IL15- activated PE-NK cells were labeled with 5 μM violet colorant (Invitrogen) before the last injection according to standard protocols, and the day after injection mice (at day 45) were scarified and tumor-infiltrating Violet+ NK cells were analyzed by flow cytometry.
Analysis of soluble factors
MICA and MICB levels were measured in PE and in the cell-free supernatants (spt) of tumor cell line cultures derived from malignant PE by a competitive enzyme-linked immunosorbent assay (ELISA) technique using a commercially available ELISA kits (MICA kit purchased from BioVendor and MICB kit purchased from Cusabio). TGFβ, IL6, IL8, IL10, IP-10, VEGF, MCP1 were detected by MILLIPLEX® MAP magnetic bead-based multi-analyte panels evaluated using Luminex Technology (MagPix, Merck Millipore, Darmstadt, Germany).
Statistical analyses
The data obtained in multiple experiments are reported as mean or percentages ± SEM (standard error of the mean). Statistical analyses were performed with GraphPad Prism 6 software (La Jolla, CA). For the statistical analysis were used: in Figs. 1C and D, 2B–D one-way ANOVA Kruskal–Wallis test; in Figs. 4A and B two-way ANOVA test; in Figs. 4C and E and Figs. 6A–C Mann–Whitney test. We considered significant p-values of less than 0.05 (*), less than 0.01 (**), less than 0.001 (***), or less than 0.0001 (****). Where not indicated, data were not statistically significant.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Eric Vivier for the generous gift of anti-B7H6 mAb and Chiara Vitale for fruitful discussion.
Funding
This work was supported by grants awarded by Associazione Italiana Ricerca sul Cancro (AIRC): IG 2010 project n.10225 (L.M.), IG 2014 project n.15283 (L.M.); Special Program Molecular Clinical Oncology 5 × 1000 project n.9962 (L.M.); 5 × 1000 Italian Ministry of Health 2013 (M.C.M.); Italian Ministry of Health, Young Researcher GR-2013–02356568, (P.V.); FRA 2015, 100008–2015-GP-FRA_001, DIMES, University of Genoa (G. Pietra); Italian Ministry of Health Ricerca Corrente 2016 (M.C.M).
References
- 1.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE et al.. Innate lymphoid cells–a proposal for uniform nomenclature. Nat Rev Immunol 2013; 13:145-9; PMID:23348417; http://dx.doi.org/ 10.1038/nri3365 [DOI] [PubMed] [Google Scholar]
- 2.Montaldo E, Vacca P, Moretta L, Mingari MC. Development of human natural killer cells and other innate lymphoid cells. Semin Immunol 2014; 26:107-13; PMID:24559836; http://dx.doi.org/ 10.1016/j.smim.2014.01.006 [DOI] [PubMed] [Google Scholar]
- 3.Eberl G, Di Santo JP, Vivier E. The brave new world of innate lymphoid cells. Nat Immunol 2015; 16:1-5; PMID:25521670; http://dx.doi.org/ 10.1038/ni.3059 [DOI] [PubMed] [Google Scholar]
- 4.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science 2011; 331:44-9; PMID:21212348; http://dx.doi.org/ 10.1126/science.1198687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moretta A, Bottino C, Vitale M, Pende D, Biassoni R, Mingari MC, Moretta L. Receptors for HLA class-I molecules in human natural killer cells. Annu Rev Immunol 1996; 14:619-48; PMID:8717527; http://dx.doi.org/ 10.1146/annurev.immunol.14.1.619 [DOI] [PubMed] [Google Scholar]
- 6.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol 2001; 19:197-223; PMID:11244035; http://dx.doi.org/ 10.1146/annurev.immunol.19.1.197 [DOI] [PubMed] [Google Scholar]
- 7.Moretta L, Bottino C, Pende D, Mingari MC, Biassoni R, Moretta A. Human natural killer cells: their origin, receptors and function. Eur J Immunol 2002; 32:1205-11; PMID:11981807; http://dx.doi.org/ 10.1002/1521-4141(200205)32:5%3c1205::AID-IMMU1205%3e3.0.CO;2-Y [DOI] [PubMed] [Google Scholar]
- 8.Moretta L, Bottino C, Pende D, Vitale M, Mingari MC, Moretta A. Different checkpoints in human NK-cell activation. Trends Immunol 2004; 25:670-6; PMID:15530838; http://dx.doi.org/ 10.1016/j.it.2004.09.008 [DOI] [PubMed] [Google Scholar]
- 9.Moretta A, Bottino C, Vitale M, Pende D, Biassoni R, Mingari MC, Moretta L. Receptors for HLA class-I molecules in human natural killer cells. Annu Rev Immunol 1996; 14:619-48; PMID:8717527; http://dx.doi.org/15771571 10.1146/annurev.immunol.14.1.619 [DOI] [PubMed] [Google Scholar]
- 10.Lanier LL. NK cell recognition. Annu Rev Immunol 2005; 23:225-74; PMID:15771571; http://dx.doi.org/ 10.1146/annurev.immunol.23.021704.115526 [DOI] [PubMed] [Google Scholar]
- 11.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 1999; 285:727-9; PMID:10426993; http://dx.doi.org/ 10.1126/science.285.5428.727 [DOI] [PubMed] [Google Scholar]
- 12.Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, Kubin M, Chalupny NJ. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity 2001; 14:123-33; PMID:11239445; http://dx.doi.org/ 10.1016/S1074-7613(01)00095-4 [DOI] [PubMed] [Google Scholar]
- 13.Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, Cantoni C, Grassi J, Marcenaro S, Reymond N et al.. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med 2003; 198:557-67; PMID:12913096; http://dx.doi.org/ 10.1084/jem.20030788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pogge von Strandmann E, Simhadri VR, von Tresckow B, Sasse S, Reiners KS, Hansen HP, Rothe A, Böll B, Simhadri VL, Borchmann P et al.. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity 2007; 27:965-74; PMID:18055229; http://dx.doi.org/ 10.1016/j.immuni.2007.10.010 [DOI] [PubMed] [Google Scholar]
- 15.Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, Haldeman B, Ostrander CD, Kaifu T, Chabannon C et al.. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med 2009; 206:1495-503; PMID:19528259; http://dx.doi.org/ 10.1084/jem.20090681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baychelier F, Sennepin A, Ermonval M, Dorgham K, Debre P, Vieillard V. Identification of a cellular ligand for the natural cytotoxicity receptor NKp44. Blood 2013; 122:2935-42; PMID:23958951; http://dx.doi.org/ 10.1182/blood-2013-03-489054 [DOI] [PubMed] [Google Scholar]
- 17.Caligiuri MA. Human natural killer cells. Blood 2008; 112:461-9; PMID:18650461; http://dx.doi.org/ 10.1182/blood-2007-09-077438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol 2001; 22:633-40; PMID:11698225; http://dx.doi.org/ 10.1016/S1471-4906(01)02060-9 [DOI] [PubMed] [Google Scholar]
- 19.De Maria A, Bozzano F, Cantoni C, Moretta L. Revisiting human natural killer cell subset function revealed cytolytic CD56(dim)CD16+ NK cells as rapid producers of abundant IFN-gamma on activation. Proc Natl Acad Sci U S A 2011; 108:728-32; PMID:21187373; http://dx.doi.org/ 10.1073/pnas.1012356108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aranda F, Buque A, Bloy N, Castoldi F, Eggermont A, Cremer I, Fridman WH, Fucikova J, Galon J, Spisek R et al.. Trial watch: Adoptive cell transfer for oncological indications. Oncoimmunology 2015; 4:e1046673; PMID:26451319; http://dx.doi.org/ 10.1080/2162402X.2015.1046673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon SR, Kim TD, Choi I. Understanding of molecular mechanisms in natural killer cell therapy. Exp Mol Med 2015; 47:e141; PMID:25676064; http://dx.doi.org/24941376 10.1038/emm.2014.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsen SK, Gao Y, Basse PH. NK cells in the tumor microenvironment. Crit Rev Oncog 2014; 19:91-105; PMID:24941376; http://dx.doi.org/ 10.1615/CritRevOncog.2014011142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vacca P, Montaldo E, Croxatto D, Moretta F, Bertaina A, Vitale C, Locatelli F, Mingari MC, Moretta L. NK cells and other innate lymphoid cells in hematopoietic stem cell transplantation. Front Immunol 2016; 7:188; PMID:27242795; http://dx.doi.org/ 10.3389/fimmu.2016.00188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burke S, Lakshmikanth T, Colucci F, Carbone E. New views on natural killer cell-based immunotherapy for melanoma treatment. Trends Immunol 2010; 31:339-45; PMID:20655806; http://dx.doi.org/ 10.1016/j.it.2010.06.003 [DOI] [PubMed] [Google Scholar]
- 25.Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, Martos JA, Moreno M. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer 1997; 79:2320-8; PMID:9191519; http://dx.doi.org/ 10.1002/(SICI)1097-0142(19970615)79:12%3c2320::AID-CNCR5%3e3.0.CO;2-P [DOI] [PubMed] [Google Scholar]
- 26.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, Aridome K, Hokita S, Aikou T. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer 2000; 88:577-83; PMID:10649250; http://dx.doi.org/ 10.1002/(SICI)1097-0142(20000201)88:3%3c577::AID-CNCR13%3e3.0.CO;2-V [DOI] [PubMed] [Google Scholar]
- 27.Villegas FR, Coca S, Villarrubia VG, Jimenez R, Chillon MJ, Jareno J, Zuil M, Callol L. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer 2002; 35:23-8; PMID:11750709; http://dx.doi.org/ 10.1016/S0169-5002(01)00292-6 [DOI] [PubMed] [Google Scholar]
- 28.Sznurkowski JJ, Zawrocki A, Biernat W. Subtypes of cytotoxic lymphocytes and natural killer cells infiltrating cancer nests correlate with prognosis in patients with vulvar squamous cell carcinoma. Cancer Immunol Immunother 2014; 63:297-303; PMID:24368339; http://dx.doi.org/ 10.1007/s00262-013-1511-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cozar JM, Canton J, Tallada M, Concha A, Cabrera T, Garrido F, Ruiz-Cabello Osuna F. Analysis of NK cells and chemokine receptors in tumor infiltrating CD4 T lymphocytes in human renal carcinomas. Cancer Immunol Immunother 2005; 54:858-66; PMID:15887015; http://dx.doi.org/ 10.1007/s00262-004-0646-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takanami I, Takeuchi K, Giga M. The prognostic value of natural killer cell infiltration in resected pulmonary adenocarcinoma. J Thorac Cardiovasc Surg 2001; 121:1058-63; PMID:11385371; http://dx.doi.org/ 10.1067/mtc.2001.113026 [DOI] [PubMed] [Google Scholar]
- 31.Carrega P, Morandi B, Costa R, Frumento G, Forte G, Altavilla G, Ratto GB, Mingari MC, Moretta L, Ferlazzo G. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(-) cells and display an impaired capability to kill tumor cells. Cancer 2008; 112:863-75; PMID:18203207; http://dx.doi.org/ 10.1002/cncr.23239 [DOI] [PubMed] [Google Scholar]
- 32.Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, Gonçalves A, André P, Romagné F, Thibault G et al.. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest 2011; 121:3609-22; PMID:21841316; http://dx.doi.org/ 10.1172/JCI45816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eckl J, Buchner A, Prinz PU, Riesenberg R, Siegert SI, Kammerer R, Nelson PJ, Noessner E. Transcript signature predicts tissue NK cell content and defines renal cell carcinoma subgroups independent of TNM staging. J Mol Med 2012; 90:55-66; PMID:21870102; http://dx.doi.org/ 10.1007/s00109-011-0806-7 [DOI] [PubMed] [Google Scholar]
- 34.Vacca P, Martini S, Garelli V, Passalacqua G, Moretta L, Mingari MC. NK cells from malignant pleural effusions are not anergic but produce cytokines and display strong antitumor activity on short-term IL-2 activation. Eur J Immunol 2013; 43:550-61; PMID:23192659; http://dx.doi.org/ 10.1002/eji.201242783 [DOI] [PubMed] [Google Scholar]
- 35.Terme M, Fridman WH, Tartour E. NK cells from pleural effusions are potent antitumor effector cells. Eur J Immunol 2013; 43:331-4; PMID:23322344; http://dx.doi.org/ 10.1002/eji.201243264 [DOI] [PubMed] [Google Scholar]
- 36.Vacca P, Martini S, Mingari MC, Moretta L. NK cells from malignant pleural effusions are potent antitumor effectors: A clue for adoptive immunotherapy? Oncoimmunology 2013; 2:e23638; PMID:23734317; http://dx.doi.org/ 10.4161/onci.23638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elkadi D, Wiernik PH, Tong TR. Resolution of massive pleural effusion due to lymphoma with intrapleural interleukin-2. Am J Hematol 2010; 85:711-2; PMID:20063279; http://dx.doi.org/ 10.1002/ajh.21604 [DOI] [PubMed] [Google Scholar]
- 38.Kammula US, White DE, Rosenberg SA. Trends in the safety of high dose bolus interleukin-2 administration in patients with metastatic cancer. Cancer 1998; 83:797-805; PMID:9708948; http://dx.doi.org/ 10.1002/(SICI)1097-0142(19980815)83:4%3c797::AID-CNCR25%3e3.0.CO;2-M [DOI] [PubMed] [Google Scholar]
- 39.Terme M, Ullrich E, Delahaye NF, Chaput N, Zitvogel L. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat Immunol 2008; 9:486-94; PMID:18425105; http://dx.doi.org/ 10.1038/ni1580 [DOI] [PubMed] [Google Scholar]
- 40.Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res J Am Assoc Cancer Res 2011; 17:6287-97; PMID:21844012; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med 2005; 202:919-29; PMID:16186184; http://dx.doi.org/ 10.1084/jem.20050463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bachanova V, Burns LJ, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lindgren BR, Cooley S, Weisdorf D, Miller JS. Allogeneic natural killer cells for refractory lymphoma. Cancer Immunol Immunother 2010; 59:1739-44; PMID:20680271; http://dx.doi.org/ 10.1007/s00262-010-0896-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodella L, Zamai L, Rezzani R, Artico M, Peri G, Falconi M, Facchini A, Pelusi G, Vitale M. Interleukin 2 and interleukin 15 differentially predispose natural killer cells to apoptosis mediated by endothelial and tumour cells. Br J Haematol 2001; 115:442-50; PMID:11703348; http://dx.doi.org/ 10.1046/j.1365-2141.2001.03055.x [DOI] [PubMed] [Google Scholar]
- 44.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A 2009; 106:1915-9; PMID:19181844; http://dx.doi.org/ 10.1073/pnas.0813192106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med 2012; 209:2351-65; PMID:23209317; http://dx.doi.org/ 10.1084/jem.20120944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jakobisiak M, Golab J, Lasek W. Interleukin 15 as a promising candidate for tumor immunotherapy. Cytokine Growth Factor Rev 2011; 22:99-108; PMID:21531164; http://dx.doi.org/12070282 10.1016/j.cytogfr.2011.04.001 [DOI] [PubMed] [Google Scholar]
- 47.Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med 2002; 195:1541-8; PMID:12070282; http://dx.doi.org/ 10.1084/jem.20020369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marcais A, Cherfils-Vicini J, Viant C, Degouve S, Viel S, Fenis A, Rabilloud J, Mayol K, Tavares A, Bienvenu J et al.. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat Immunol 2014; 15:749-57; PMID:24973821; http://dx.doi.org/ 10.1038/ni.2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szczepanski MJ, Szajnik M, Welsh A, Foon KA, Whiteside TL, Boyiadzis M. Interleukin-15 enhances natural killer cell cytotoxicity in patients with acute myeloid leukemia by upregulating the activating NK cell receptors. Cancer Immunol Immunother 2010; 59:73-9; PMID:19526239; http://dx.doi.org/ 10.1007/s00262-009-0724-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dulphy N, Chretien AS, Khaznadar Z, Fauriat C, Nanbakhsh A, Caignard A, Chouaib S, Olive D, Toubert A. Underground Adaptation to a Hostile Environment: Acute Myeloid Leukemia vs. Natural Killer Cells Front Immunol 2016; 7:94; PMID:27014273; http://dx.doi.org/25403209 10.3389/fimmu.2016.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, Fleisher TA, Dubois SP, Perera LP, Stewart DM et al.. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol J Am Clin Oncol 2015; 33:74-82; PMID:25403209; http://dx.doi.org/ 10.1200/JCO.2014.57.3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vacchelli E, Aranda F, Bloy N, Buque A, Cremer I, Eggermont A, Fridman WH, Fucikova J, Galon J, Spisek R et al.. Trial Watch-Immunostimulation with cytokines in cancer therapy. Oncoimmunology 2016; 5:e1115942; PMID:27057468; http://dx.doi.org/ 10.1080/2162402X.2015.1115942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tarhini AA, Millward M, Mainwaring P, Kefford R, Logan T, Pavlick A, Kathman SJ, Laubscher KH, Dar MM, Kirkwood JM. A phase 2, randomized study of SB-485232, rhIL-18, in patients with previously untreated metastatic melanoma. Cancer 2009; 115:859-68; PMID:19140204; http://dx.doi.org/ 10.1002/cncr.24100 [DOI] [PubMed] [Google Scholar]
- 54.Ardolino M, Azimi CS, Iannello A, Trevino TN, Horan L, Zhang L, Deng W, Ring AM, Fischer S, Garcia KC et al.. Cytokine therapy reverses NK cell anergy in MHC-deficient tumors. J Clin Invest 2014; 124:4781-94; PMID:25329698; http://dx.doi.org/ 10.1172/JCI74337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krieg S, Ullrich E. Novel immune modulators used in hematology: impact on NK cells. Front Immunol 2012; 3:388; PMID:23316191; http://dx.doi.org/ 10.3389/fimmu.2012.00388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Romagne F, Andre P, Spee P, Zahn S, Anfossi N, Gauthier L, Capanni M, Ruggeri L, Jr Benson DM, Blaser BW et al.. Preclinical characterization of 1–7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood 2009; 114:2667-77; PMID:19553639; http://dx.doi.org/ 10.1182/blood-2009-02-206532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kohrt HE, Thielens A, Marabelle A, Sagiv-Barfi I, Sola C, Chanuc F, Fuseri N, Bonnafous C, Czerwinski D, Rajapaksa A et al.. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood 2014; 123:678-86; PMID:24326534; http://dx.doi.org/ 10.1182/blood-2013-08-519199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vahlne G, Lindholm K, Meier A, Wickstrom S, Lakshmikanth T, Brennan F, Wilken M, Nielsen R, Romagné F, Wagtmann NR et al.. In vivo tumor cell rejection induced by NK cell inhibitory receptor blockade: maintained tolerance to normal cells even in the presence of IL-2. Eur J Immunol 2010; 40:813-23; PMID:20039300; http://dx.doi.org/ 10.1002/eji.200939755 [DOI] [PubMed] [Google Scholar]
- 59.Pilipow K, Roberto A, Roederer M, Waldmann TA, Mavilio D, Lugli E. IL15 and T-cell stemness in t-cell-based cancer immunotherapy. Cancer Res 2015; 75:5187-93; PMID:26627006; http://dx.doi.org/ 10.1158/0008-5472.CAN-15-1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leong JW, Chase JM, Romee R, Schneider SE, Sullivan RP, Cooper MA, Fehniger TA. Preactivation with IL-12, IL-15, and IL-18 induces CD25 and a functional high-affinity IL-2 receptor on human cytokine-induced memory-like natural killer cells. Biol Blood Marrow Transplan J Am Soc Blood Marrow Transplan 2014; 20:463-73; PMID:24434782; http://dx.doi.org/ 10.1016/j.bbmt.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rezvani K, Rouce RH. The Application of Natural Killer Cell Immunotherapy for the Treatment of Cancer. Front Immunol 2015; 6:578; PMID:26635792; http://dx.doi.org/ 10.3389/fimmu.2015.00578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Romagnani C, Juelke K, Falco M, Morandi B, D'Agostino A, Costa R, Ratto G, Forte G, Carrega P, Lui G et al.. CD56(bright) CD16(-) killer Ig-like receptor(-) NK cells display longer telomeres and acquire features of CD56(dim) NK cells upon activation. J Immunol 2007; 178:4947-55; PMID:17404276; http://dx.doi.org/2784720 10.4049/jimmunol.178.8.4947 [DOI] [PubMed] [Google Scholar]
- 63.Hasui M, Saikawa Y, Miura M, Takano N, Ueno Y, Yachie A, Miyawaki T, Taniguchi N. Effector and precursor phenotypes of lymphokine-activated killer cells in mice with severe combined immunodeficiency (scid) and athymic (nude) mice. Cell Immunol 1989; 120:230-9; PMID:2784720; http://dx.doi.org/ 10.1016/0008-8749(89)90190-1 [DOI] [PubMed] [Google Scholar]
- 64.Vitale M, Cantoni C, Pietra G, Mingari MC, Moretta L. Effect of tumor cells and tumor microenvironment on NK-cell function. Eur J Immunol 2014; 44:1582-92; PMID:24777896; http://dx.doi.org/ 10.1002/eji.201344272 [DOI] [PubMed] [Google Scholar]
- 65.Pietra G, Vitale C, Pende D, Bertaina A, Moretta F, Falco M, Vacca P, Montaldo E, Cantoni C, Mingari MC et al.. Human natural killer cells: news in the therapy of solid tumors and high-risk leukemias. Cancer Immunol Immunother 2016; 65:465-76; PMID:26289090; http://dx.doi.org/ 10.1007/s00262-015-1744-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011; 29:235-71; PMID:21219185; http://dx.doi.org/ 10.1146/annurev-immunol-031210-101324 [DOI] [PubMed] [Google Scholar]
- 67.Moretta L, Pietra G, Montaldo E, Vacca P, Pende D, Falco M, Del Zotto G, Locatelli F, Moretta A, Mingari MC. Human NK Cells: From Surface Receptors to the Therapy of Leukemias and Solid Tumors. Front Immunol 2014; 5:87; PMID:24639677; http://dx.doi.org/ 10.3389/fimmu.2014.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12:298-306; PMID:22419253; http://dx.doi.org/ 10.1038/nrc3245 [DOI] [PubMed] [Google Scholar]
- 69.Fridman WH, Galon J, Pages F, Tartour E, Sautes-Fridman C, Kroemer G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res 2011; 71:5601-5; PMID:21846822; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-1316 [DOI] [PubMed] [Google Scholar]
- 70.Sconocchia G, Spagnoli GC, Del Principe D, Ferrone S, Anselmi M, Wongsena W, Cervelli V, Schultz-Thater E, Wyler S, Carafa V et al.. Defective infiltration of natural killer cells in MICA/B-positive renal cell carcinoma involves beta(2)-integrin-mediated interaction. Neoplasia 2009; 11:662-71; PMID:19568411; http://dx.doi.org/ 10.1593/neo.09296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Halama N, Braun M, Kahlert C, Spille A, Quack C, Rahbari N, Koch M, Weitz J, Kloor M, Zoernig I et al.. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin Cancer Res J Am Assoc Cancer Res 2011; 17:678-89; PMID:21325295; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-2173 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.