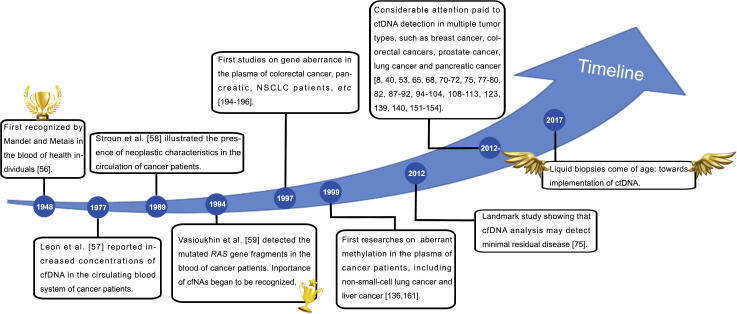
Figure 1.
Landmarks in the detection of ctDNAs in patients with different cancers
This timeline shows the development of ctDNA detection of genetic and epigenetic alterations. Since the first validation of ctDNAs in 1948 [56], increasing interest has been attracted due to its ability for detection and broad clinical applicability. In 1977, Leon et al. [57] found increased concentrations of cfDNAs circulating in cancer patients. Ten years later, Stroun et al. [58] illustrated the presence of neoplastic characteristics in the circulation of cancer patients. The importance of cfNAs began to be recognized around the year 1994 [59]. At the time, the first studies on aberrant genetic alterations [194], [195], [196] and methylations [136], [161] were of high interest to the public. In 2012, a landmark study by Shaw et al. [75] showed that analyses of cfDNAs may help to detect minimal residual disease. cfDNA, cell-free DNA; cfNA, cell-free nucleic acid; ctDNA, circulating tumor DNA; NSCLC, non-small-cell lung cancer.