Figure 1.
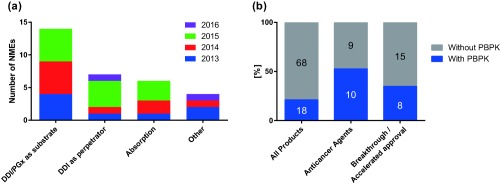
Overview of physiologically based pharmacokinetic (PBPK) information in product labels or US Food and Drug Administration (FDA) review documents for drugs approved by the FDA between January 2013 and August 2016. (a) Number of new molecular entities (NMEs) with information of PBPK for respective areas of applications. (b) Proportion of product labels/reviews containing PBPK information for drugs in all NMEs, anticancer agents, and NMEs with breakthrough therapy designation and/or accelerated approval status at the time of approval. The numbers on the bars represent the number of products in each category. Three of eight NMEs with PBPK in nononcology field were for rare diseases. Seven of eight NMEs with PBPK and breakthrough/accelerated approval status were anticancer agents. DDI, drug‐drug interaction; PGx, pharmacogenomics.
