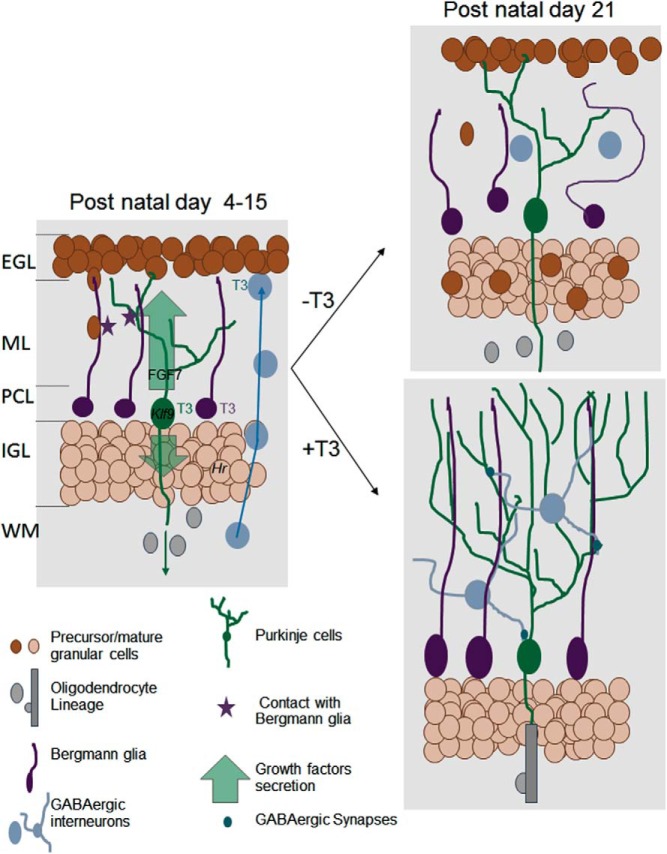
Figure 2.
Direct and indirect influence of T3 on postnatal cerebellum development. The cerebellum cortex has a layered structured (EGL, external granular layer; ML, molecular layer; PCL, Purkinje cells layer; IGL, inner granular layer; WM, white matter). During early postnatal development (left), T3 cell-autonomous function is mainly to activate the differentiation of Purkinje cells (green), Bergmann glia (the cerebellum radial astrocytes, in purple), and probably also γaminobutyric acid (GABA)ergic interneurons (blue). The underlying genetic regulation is, for a large part, still unknown. Among few well-characterized TR target genes are Klf9, a transcription factor involved in Purkinje cells maturation, and Hr, a gene encoding a transcription corepressor in postmitotic granule cells the neurodevelopmental function of which is unclear. The T3-stimulated Purkinje cells secrete a number of neurotrophins and growth factors, including FGF7. These secreted factors stimulate myelin formation by oligodendrocytes in the WM and the cell-cycle exit of granule cell precursors in EGL. Direct contacts with Bergmann glia fibers guide granule cells inward migration. They also favor the establishment of synapses between Purkinje cells and GABAergic interneurons. Three weeks after birth (right, bottom), the terminal differentiation of all cell types and synaptogenesis are normally achieved. Purkinje cells axons are myelinated and EGL has disappeared. In absence of T3 stimulation (right, top), Purkinje cell arborization is reduced, Bergmann glia extension are disorganized, and cell bodies are not anchored in the PCL. Granule cell precursors migration is impaired. They accumulate in EGL and eventually die. Terminal differentiation of GABAergic interneurons is also impaired, and density of GABAergic synapses is low. In the WM, oligodendrocytes differentiation and myelin formation are delayed. FGF, fibroblast growth factor.