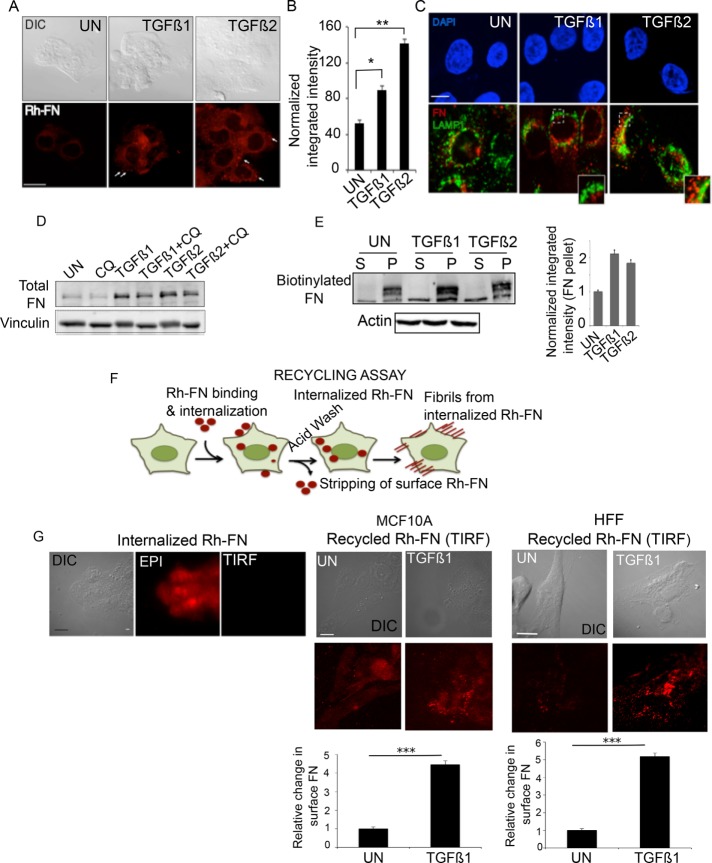
FIGURE 4:
TGF-β increases FN internalization and recycling for fibrillogenesis. (A) Rh-FN at 20 µg/ml was added to MCF10A cells either untreated or in the presence of TGF-β1 and TGF-β2 for 30 min, followed by stripping of cell surface Rh-FN (Materials and Methods). Representative fluorescence images of Rh-FN. Scale bar, 5 µm. Arrows indicate internalized Rh-FN. (B) Quantification of integrated fluorescence intensity of Rh-FN normalized to cell area from cells in A; N > 100/condition. Significance was calculated between untreated samples and TGF-β1– (*p < 0.05) and TGF-β2–treated (**p < 0.01) samples using the Mann–Whitney U test. Data are representative of at least three independent experiments. (C) MCF10A cells treated with TGF-β1/TGF-β2 (10 ng/ml, 30 min) were coimmunostained using anti-LAMP1 and anti-FN; images acquired using confocal microscopy. Scale bar, 5 µm. (D) MCF10A cells were treated with TGF-β1 or TGF-β2 for 6 h with or without 100 µM CQ. Lysates were immunoblotted for total FN. Vinculin was used as loading control. (E) Representative DOC-fractionated biotinylated FN (FN-biotin; Materials and Methods) in MCF10A cells. FN-biotin at 20 µg/ml was added to cells for 30 min and washed to remove nonspecific FN-biotin binding on the cell surface. The cells were then reincubated in FN-biotin–free medium for 1 h with or without TGF-β1 or TGF-β2, DOC fractionated into (S) and (P) fractions, and immunoblotted using streptavidin-conjugated IR dye to detect FN-biotin. Actin was used as the loading control for the (S) fraction. Right, quantitation showing fold difference between the amounts of FN-biotin in the DOC-fractionated (P) fraction in untreated and TGF-β1– or TGF-β2–treated cells. Data represent quantitation from two independent experiments. Error bars represent SEM. (F) Schematic of the recycling assay using Rh-FN upon incubation of cells with 20 µg/ml Rh-FN for 30 min at 37°C in the absence of TGF-β1 or TGF-β2. Cells are acid stripped to remove surface-bound Rh-FN (Internalized Rh-FN), followed by reincubation of cells in Rh-FN–free medium at 37°C for 1 h in the presence of TGF-β1 or TGF-β2. (G) Recycling assay as described in F; left, Rh-FN internalized by cells imaged after acid stripping using the Epi illumination and TIRF modes (90-nm penetration depth) as indicated to confirm removal of cell surface Rh-FN. Right, images taken under TIRF mode in either MCF10A or HFF cells, showing the reappearance of surface FN in cells untreated or treated with TGF-β1. Images are representative of at least three independent trials. Scale bar, 5 µm. Quantitation presented below was carried out using the 3D object counter on ImageJ as described in Materials and Methods after subtracting background from the cell-free regions. N = 10/condition. ***p < 0.001.