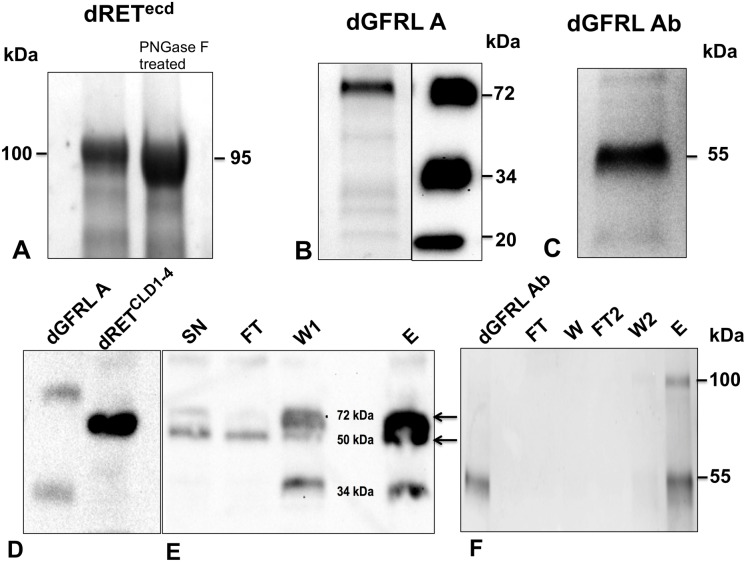
Fig 1. The BEVS successfully expressed secreted and folded fruit fly RET and GFR-like A and Ab proteins.
(A) purified dRETecd on a Coomassie brilliant blue stained gel reveals that native dRETecd (-) migrated higher than PNGase F treated dRETecd (+). (B) (left panel) purified dGFRLA on a Coomassie brilliant blue stained gel reveals that it migrated at a molecular weight of 72 kDa (right panel) and anti-Flag Western blotting identified possible proteolytic cleavage fragments of dGFRLA at 34 kDa and 20 kDa. (C) purified dGFRLAb migrated on a Coomassie brilliant blue stained gel at a molecular weight of 55 kDa. (D) Anti-Flag Western blotting showing dGFRLA and dRETCLD1-4 controls and (E), pull-down of co-transfected dGFRLA and dRETCLD1-4 where most of the dRETCLD1-4 was present in the elution. (F), Coomassie stained gel of a pull-down assay using His-tagged dGFRLAb and untagged dRETecd, where dRETecd was only seen in the elution. SN, supernatant; FT, flow-through; W, wash; E, elution.