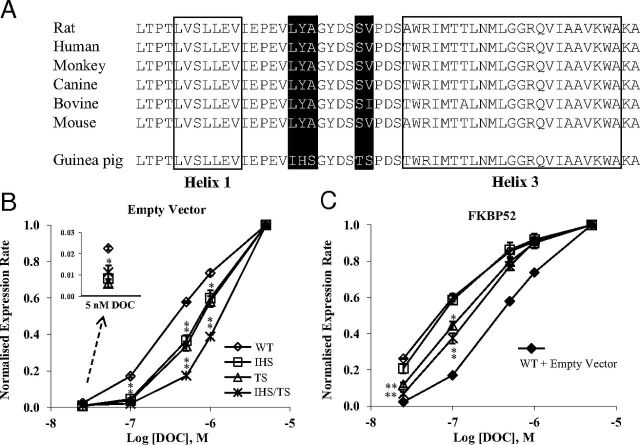
Figure 1.
Influence of the nonconservative mutations within the H1-H3 loop in gpGR on FKBP52-mediated potentiation of rGR. A, The GR LBD sequences from helix 1 to helix 3 of the rat (CAA72938), human (NP_001018087), monkey (ABM91989), canine (XP_535225.2), bovine (NP_001193563.1), mouse (NP_032199.3), and guinea pig (NP_001166458) were aligned using the ClustalW program. The amino acids constituting the 2 helices are boxed, and the nonconservative residues in the gpGR are highlighted in black. B and C, The normalized rGR reporter expression rate in the absence (B) and presence (C) of FKBP52. Yeast was transformed with a β-galactosidase reporter pUCΔSS-26x, an expression plasmid containing the wild-type (WT) or mutated rGR, and p423GPD/hFKBP52 expression plasmid or empty vector control. Aliquots of yeast culture were dosed with increasing concentrations of deoxycorticosterone (DOC). The reporter expression rate of WT and mutated rGR were normalized and presented as a function of the log of DOC concentration. Graphs represent the mean normalized expression rate ± SEM of 3 independent experiments. Inset, activity levels in the presence of 5 nM DOC. *, P < .05; **, P ≤ .001; for comparison of normalized reporter expression rate of mutant rGR with that of wild-type rGR. Note, the titration curve for wild-type receptor in the absence of FKBP52 has been included in C to illustrate the level of FKBP52 potentiation.