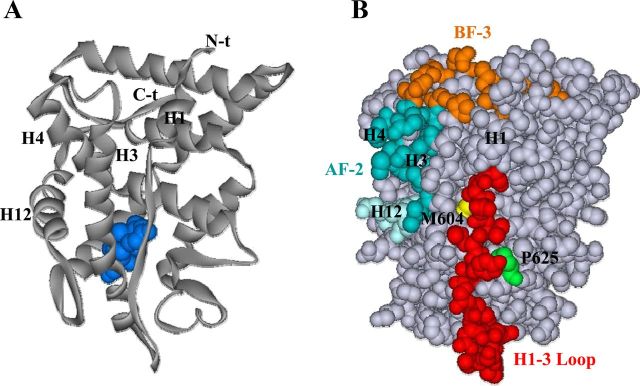
Figure 7.
Relative location of the GR H1-H3 loop to AF-2 and BF-3 surfaces and selected residues important for GR function. A, Schematic of GR LBD showing location of dexamethasone ligand (blue), H1, and AF-2 helices H3, H4, and H12. B, Space-filling model showing GR H1-H3 loop residues (red); AF-2 (H3 and H4 residues, blue; H12 residues, light blue); BF-3 regulatory surface (including conserved, partially conserved, and retained polar and hydrophobic residues identified for AR BF-3 highlighted in Figure 2A, orange); Pro625, important for GR-Hsp90 stability and nuclear localization (green); and Met604, involved in GR dependence on Hsp90 and in transmitting hormone-induced conformational changes to the GR LBD surface (yellow). Structural data were obtained from the RCSB (Research Collaboratory for Structural Bioinformatics, www.pdb.org) protein data bank (PDB ID 1P93) (89). Images were generated using the ViewerLite version 5.0 program.