Abstract
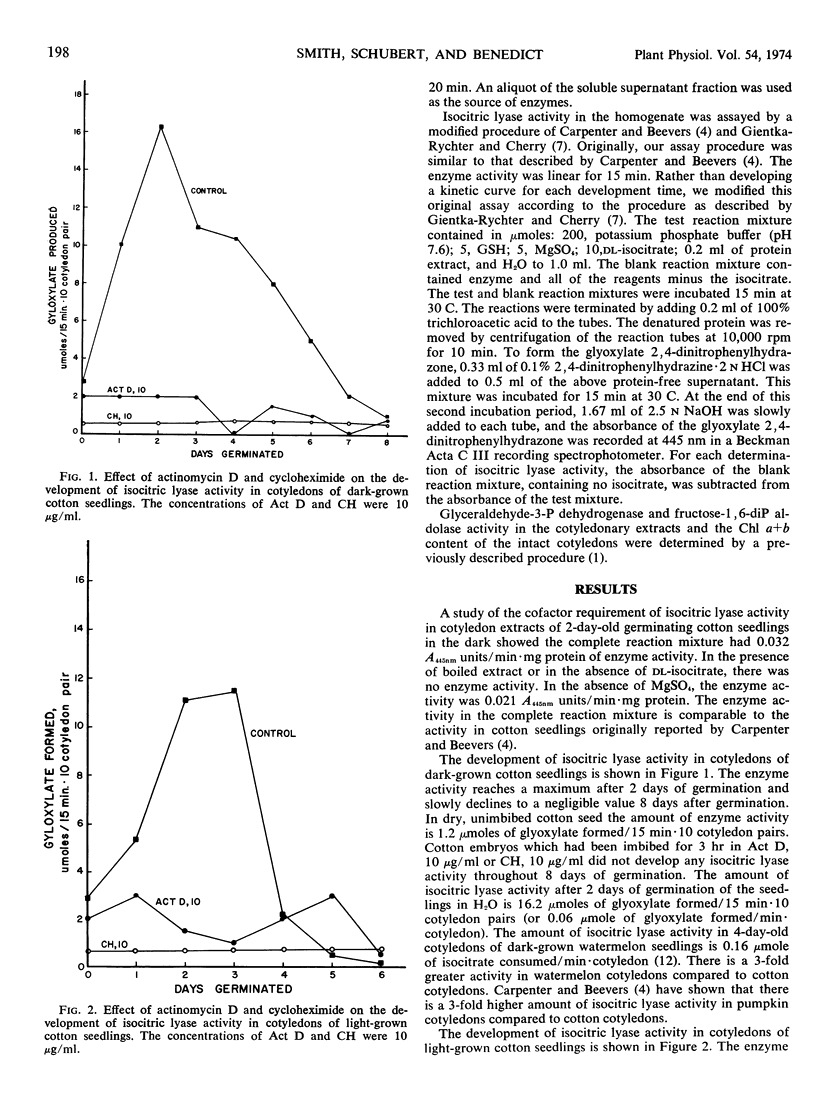
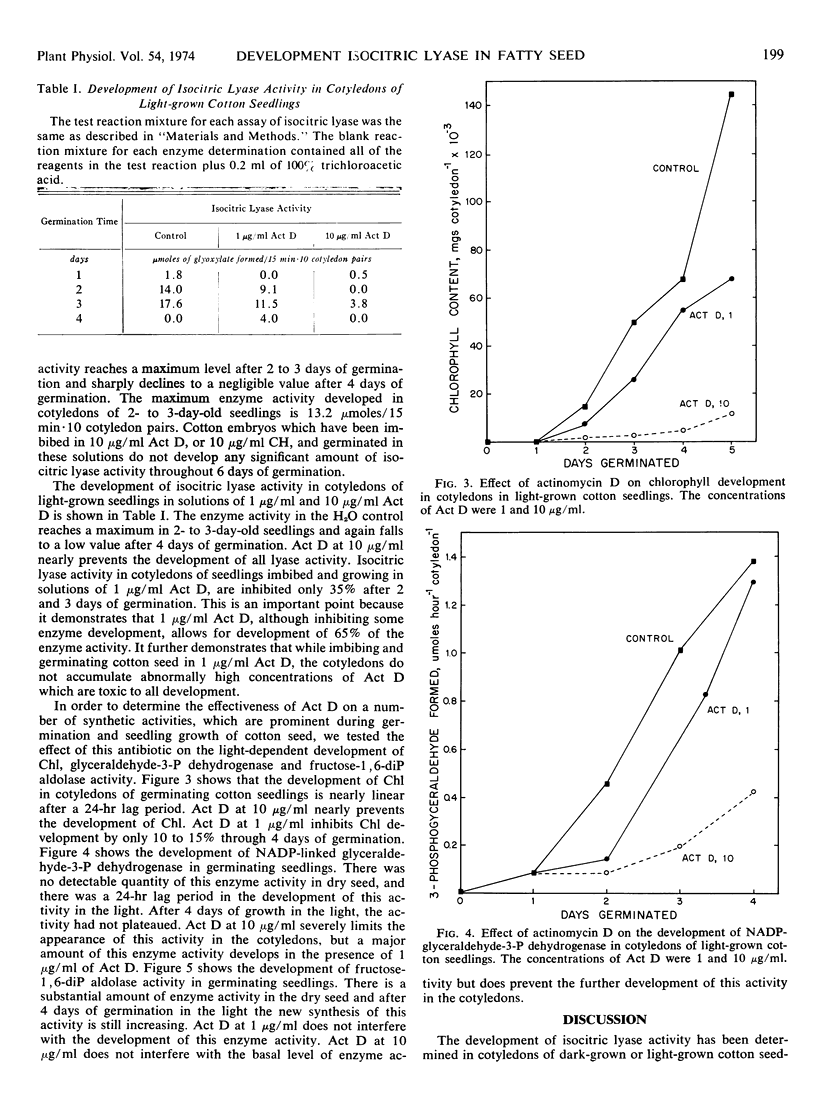
In cotyledons of germinating cotton (Gossypium hirsutum L. var. Stoneville 213) seedlings, in the dark, isocitric lyase (EC 4.1.3.1) activity peaks after 2 days and thereafter slowly declines to a negligible value after 8 days. The maximum activity of this enzyme in cotyledons of 2-day-old seedlings was 16.2 μmoles of glyoxylate formed/15 min·10 cotyledon pairs. Actinomycin D at a concentration of 10 μg/ml, if added to the imbibing solution, completely prevents the development of isocitric lyase activity in these germinating seed. In cotyledons of germinating cotton seedlings, in the light, isocitric lyase activity peaks after 2 to 3 days and sharply declines to a negligible value after 4 days. The maximum activity of this enzyme in cotyledons of 2- to 3-day-old seedlings was 13.2 μmoles of glyoxylate formed/15 min·10 cotyledon pairs. Actinomycin D at a concentration of 10 μg/ml, if added to the imbibing solution, severely inhibits the development of enzyme activity.
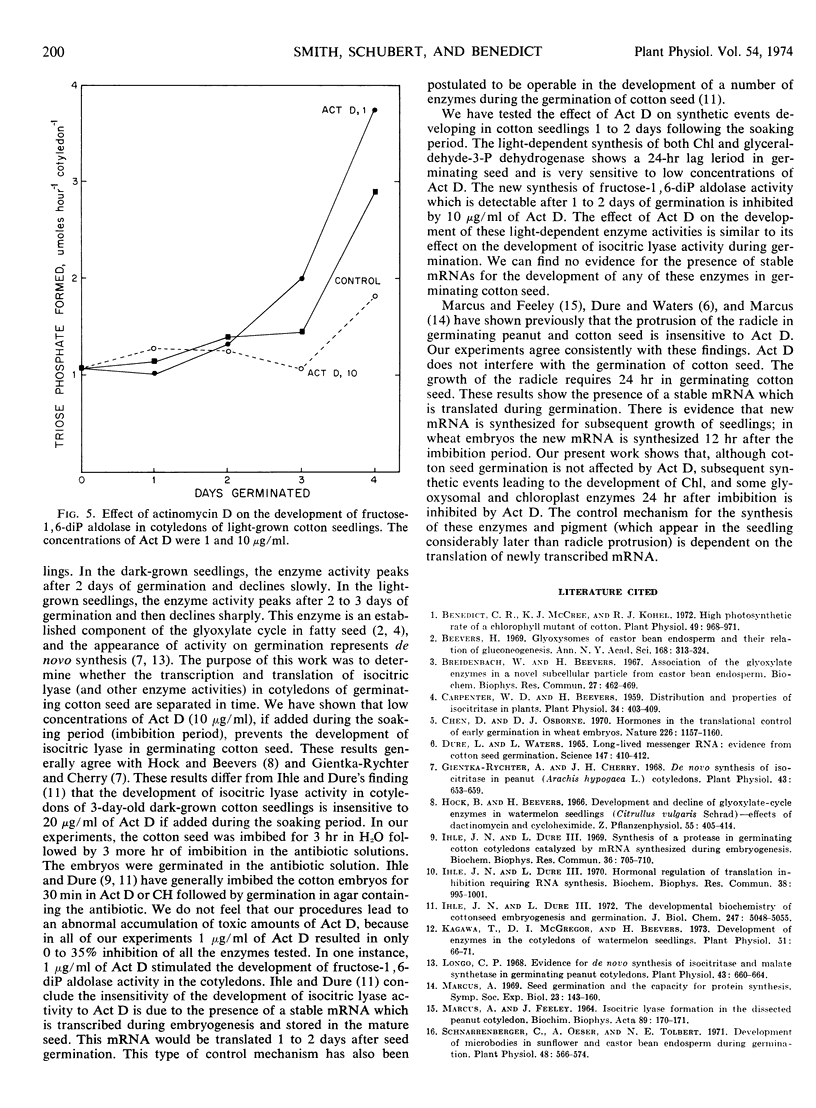
In germinating seed, in the light, the synthesis of chlorophyll and glyceraldehyde-3-P dehydrogenase is also limited by the addition of low concentrations of actinomycin D. The new synthesis of fructose-1, 6-diP aldolase, which is detectable after 1 to 2 days of germination, is inhibited by 10 μg/ml of actinomycin D. We, therefore, conclude that the synthetic events leading to the development of chlorophyll, some glyoxysomal and chloroplast enzymes in germinating cotton seedlings depend on newly transcribed mRNA.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beevers H. Glyoxysomes of castor bean endosperm and their relation to gluconeogenesis. Ann N Y Acad Sci. 1969 Dec 19;168(2):313–324. doi: 10.1111/j.1749-6632.1969.tb43118.x. [DOI] [PubMed] [Google Scholar]
- Benedict C. R., McCree K. J., Kohel R. J. High photosynthetic rate of a chlorophyll mutant of cotton. Plant Physiol. 1972 Jun;49(6):968–971. doi: 10.1104/pp.49.6.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breidenbach R. W., Beevers H. Association of the glyoxylate cycle enzymes in a novel subcellular particle from castor bean endosperm. Biochem Biophys Res Commun. 1967 May 25;27(4):462–469. doi: 10.1016/s0006-291x(67)80007-x. [DOI] [PubMed] [Google Scholar]
- Carpenter W. D., Beevers H. Distribution and Properties of Isocitritase in Plants. Plant Physiol. 1959 Jul;34(4):403–409. doi: 10.1104/pp.34.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Osborne D. J. Hormones in the translational control of early germination in wheat embryos. Nature. 1970 Jun 20;226(5251):1157–1160. doi: 10.1038/2261157a0. [DOI] [PubMed] [Google Scholar]
- DURE L., WATERS L. LONG-LIVED MESSENGER RNA: EVIDENCE FROM COTTON SEED GERMINATION. Science. 1965 Jan 22;147(3656):410–412. doi: 10.1126/science.147.3656.410. [DOI] [PubMed] [Google Scholar]
- Gientka-Rychter A., Cherry J. H. De Novo Synthesis of Isocitritase in Peanut (Arachis hypogaea L.) Cotyledons. Plant Physiol. 1968 Apr;43(4):653–659. doi: 10.1104/pp.43.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Dure L. S., 3rd The developmental biochemistry of cottonseed embryogenesis and germination. 3. Regulation of the biosynthesis of enzymes utilized in germination. J Biol Chem. 1972 Aug 25;247(16):5048–5055. [PubMed] [Google Scholar]
- Ihle J. N., Dure L., 3rd Hormonal regulation of translation inhibition requiring RNA synthesis. Biochem Biophys Res Commun. 1970 Mar 27;38(6):995–1001. doi: 10.1016/0006-291x(70)90338-4. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Dure L., 3rd Synthesis of a protease in germinating cotton cotyledons catalzed by mRNA synthesized during embryogenesis. Biochem Biophys Res Commun. 1969 Aug 22;36(5):705–710. doi: 10.1016/0006-291x(69)90667-6. [DOI] [PubMed] [Google Scholar]
- Kagawa T., McGregor D. I., Beevers H. Development of enzymes in the cotyledons of watermelon seedlings. Plant Physiol. 1973 Jan;51(1):66–71. doi: 10.1104/pp.51.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo C. P. Evidence for de novo synthesis of isocitratase and malate synthesis in germinating peanut cotyledons. Plant Physiol. 1968 Apr;43(4):660–664. doi: 10.1104/pp.43.4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCUS A., FEELEY J. ISOCITRIC LYASE FORMATION IN THE DISSECTED PEANUT COTYLEDON. Biochim Biophys Acta. 1964 Jul 8;89:170–171. doi: 10.1016/0926-6569(64)90116-6. [DOI] [PubMed] [Google Scholar]
- Marcus A. Seed germination and the capacity for protein synthesis. Symp Soc Exp Biol. 1969;23:143–160. [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Development of Microbodies in Sunflower Cotyledons and Castor Bean Endosperm during Germination. Plant Physiol. 1971 Nov;48(5):566–574. doi: 10.1104/pp.48.5.566. [DOI] [PMC free article] [PubMed] [Google Scholar]


