Figure 1.
Expression of a Minimal GlcNAc-1-Phosphotransferase and Analysis of Enzyme Activity
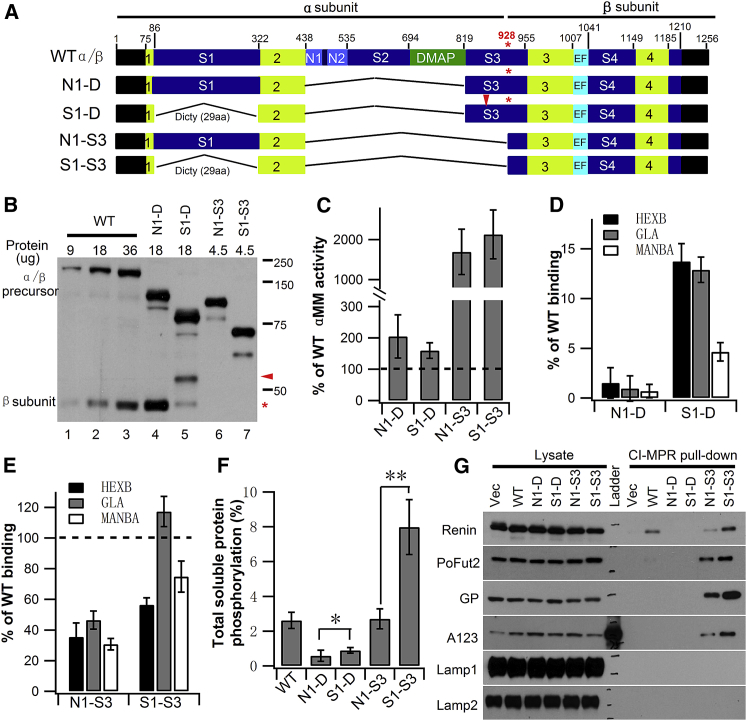
(A) Schematic of WT and the various α/β precursor deletion constructs expressed in GNPTAB−/− HeLa cells. The four regions in lime comprise the catalytic Stealth domain, while the two Notch modules (N1 and N2) and the DNA methyl-transferase-associated protein (DMAP) interaction domain are involved in lysosomal enzyme recognition. In S1-D and S1-S3, the 236 aa human spacer-1 (S1) sequence was replaced with 29 aa of the D. discoideum sequence, in addition to removal of N1 through most of spacer-3 (S3) up to the K928 cleavage site (asterisk). (B) Immunoblot of WT α/β precursor and the deletion mutants expressed in GNPTAB−/− HeLa cells. The indicated amount of each cell extract was loaded, and the α/β precursor and β subunits were detected with an anti-V5 antibody. (C) Catalytic activity of WT α/β precursor and the deletion mutants toward αMM using equal amounts of whole-cell extracts. The vector-only transfected GNPTAB−/− HeLa cell extract served as a control, and WT value was set to 100% after subtraction of vector-only background. (D and E) Transfection of GNPTAB−/− HeLa cells with either WT α/β precursor or the various deletion mutant cDNAs. The degree of phosphorylation mediated WT or mutant proteins was determined by binding of three endogenous lysosomal enzymes to CI-MPR-affinity beads. Bound material was assayed for activity, and values obtained with cells transfected with WT α/β are set to 100%. (F) Mannose phosphorylation of total soluble proteins was determined by transfecting GNPTAB−/− HeLa cells with WT α/β precursor or the indicated deletion mutant cDNAs, followed by [2-3H]mannose labeling. Values shown are calculated as the percentage of counts recovered with the CI-MPR affinity beads as a fraction of the total counts in the phosphotungstic acid precipitate. The background value of 0.8 ± 0.3% was subtracted to yield the final depicted vales. *p < 0.05, **p < 0.01. (G) Immunoblot analysis of GNPTAB−/− HeLa cells co-transfected with the expression plasmids for the indicated proteins along with empty vector, WT α/β precursor, or the indicated deletion mutant cDNAs. Cell lysates were incubated with CI-MPR-affinity beads, and the binding of the various proteins was determined by probing the blots with the following antibodies: anti-HA for Renin, anti-myc for PoFut2, anti-Strep tag for the vWF A1A2A3 domains, and antibodies generated against the native protein for GP, Lamp1, and Lamp2. Error bars represent mean ± SD.